Page 75 - Elements of Chemical Reaction Engineering 3rd Edition
P. 75
Sec. 2.3 Applications of the Design Equations for Contfnuous-Flow Reactors 47
Tlhis is also the area of the rectangle with vertices (X, l/-rA) of (0, 0), (0, 400),
(0.6, 400), and (0.6,O). The CSTR volume necessary to achieve 60% conversion is
mol 1
5 mol 240 dm3.s = 1200 dm3
v=(-)(
For the plug-flow (tubular) reactor:
dX
F -=-r (2-13
AodV A
Integrating and rearranging Equation (2-1 5) yields
0.3 .
= X [ 189 + 4(222) + 4001
dm3.s
= 148 -
mol
The PFR volume necessary to achieve 60% conversion is
[
V = pF1) dm3 s = 740 dm3
148
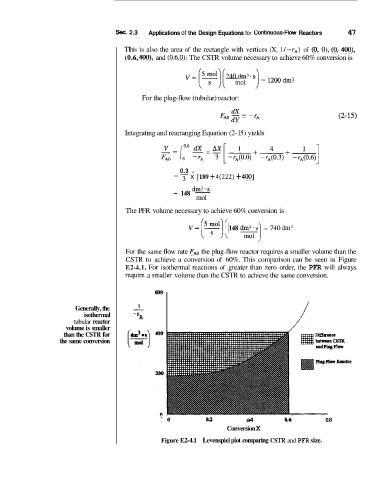
For the same flow rate FA,o the plug-flow reactor requires a smaller volume than the
CSTR to achieve a conversion of 60%. This comparison can be seen in Figure
E2-4.1. For isothermal reactions of greater than zero order, the PFR will always
rquire a srrtaller volume than the CSTR to achieve the same conversion.
6oa
1
Generally, the -
isothermal -r A i
tubular reactor
volume is smaller
than the CSTR for
the same conversion
"0 0.2 a4 a6 0.8
Conversion X
Figure E2-4.1 Levenspiel plot comparing CSTR and PFR size.