Page 87 - Elements of Chemical Reaction Engineering 3rd Edition
P. 87
Chap. 2 Summary 59
Equation (2-26) is a form of the design equation for constant volumetric
flow rate u,, that may prove more useful in determining the space time or reac-
tor volume for reaction rates that depend only on the concentration of one
species.
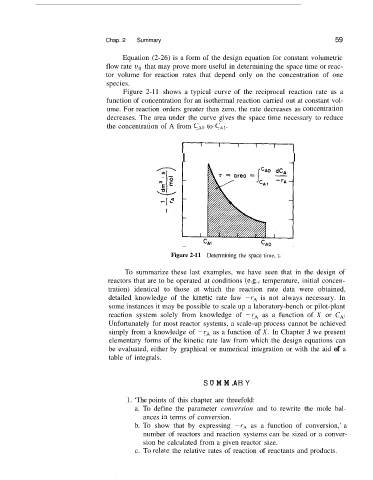
Figure 2-11 shows a typical curve of the reciprocal reaction rate as a
function of concentration for an isothermal reaction carried out at constant vol-
ume. For reaction orders greater than zero, the rate decreases as concentral.ion
decreases. The area under the curve gives the space time necessary to reduce
the concentration of A from CAo to CAI.
I I
Figure 2-11 Determining the space time, z.
To summarize these last examples, we have seen that in the design of
reactors that are to be operated at conditions (e.g., temperature, initial concen-
tration) identical to those at which the reaction rate data were obtained,
detailed knowledge of the lunetic rate law -r, is not always necessary. In
some instances it may be possible to scale up a laboratory-bench or pilot-plant
reaction system solely from knowledge of -rA as a function of X or CA.
Unfortunately for most reactor systems, a scale-up process cannot be achieved
simply from a knowledge of -rA as a function of X. In Chapter 3 we present
elementary forms of the kinetic rate law from which the design equations can
be evaluated, either by graphical or numerical integration or with the aid of a
table of integrals.
S U M M .A R Y
1. ‘The points of this chapter are threefold:
a. To define the parameter conversion and to rewrite the mole bal-
ances iin terms of conversion.
b. To show that by expressing -rA as a function of conversion,’ a
number of reactors and reaction systems can be sized or a conver-
sion be calculated from a given reactor size.
c. To relale the relative rates of reaction of reactants and products.