Page 93 - Elements of Chemical Reaction Engineering 3rd Edition
P. 93
Chap. 2 Questions and Problems 65
P2-9B The irreversible gas-phase nonelementary reaction
A+2B +
C
is to be carried out isothermally in a constant-pressure batch reactoi The feed
is at a temperature of 227"C, a presrure of 1013 kPa, and its composition IS
33.3% -4 and 66.7% &. Laboratory data taKen unaer identical conditions are
as follows (note that at Y -= 0, -rA = 0.00001):
-rA (mol/dm3.s)x IO3 0.0lll 0.005 0.002 0.OOi
X 0.0 0.2 0.4 0.6
P2-10, Estimate the reactor volumes of the two CSTRs and the PFR shown in
Figure 2-7.
P2-llr, Don't calculate anything. Just go home and relax.
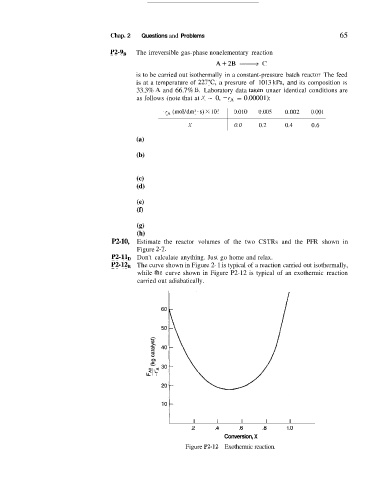
P2-12B The curve shown in Figure 2- 1 is typical of a reaction carried out isothermally,
while tbe curve shown in Figure P2-12 is typical of an exothermic reaction
carried out adiabatically.
I I I I I
.2 .4 .6 .a 1 .o
Conversion, X
Figure P?-12 Exothermic reaction.