Page 190 - Elements of Chemical Reaction Engineering Ebook
P. 190
Sec. 4..4 Pressure Drop in Reactors 161
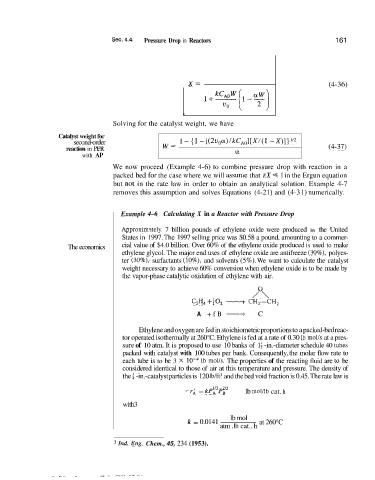
X= (4-36)
I
Solving for the catalyst weight, we have
Catalyst weight for
-
second-order 1 - {I - [(2uoff)/kC,,][X/(1 X)]}”Z
reaction in PER W= (4-37)
with AP o!
We now proceed (Example 4-6) to combine pressure drop with reaction in a
packed bed for the case where we will assunle that EX 4 1 in the Ergun equation
but nolt in the rate law in order to obtain an analytical solution. Example 4-7
removes this assumption and solves Equations (4-21 ) and (4-3 1) numerically.
Example 46 Calculating X in a Reactor with Pressure Drop
Approxim.*tely 7 billion pounds of ethylene oxide were produced in the United
States in 1997. The 1997 selling price was $0.58 a pound, amounting to a commer-
The economics cial value of $4.0 billion. Over 60% of the ethylene oxide produced is used to make
ethylene glycol. The major end uses of ethylene oxide are antifreeze (30%), polyes-
ter (30%), surfactants (lo%), and solvents (5%). We want to calculate the catalyst
weight necessary to achieve 60% conversion when ethylene oxide is to be made by
the vapor-phase catalytic oxidation of ethylene with air.
/O\
C2H4 + i02 ___f CH2-CH,
A +fB ---+ C
Ethylene and oxygen are fed in stoichiometric proportions to a packed-bed reac-
tor operated isothermally at 260°C. Ethylene is fed at a rate of 0.30 Ib mol/s at a pres-
sure of 10 atm. It is proposed to use 10 banks of 1 f -in.-diameter schedule 40 tubes
packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to
each tube is to be 3 X Ib mol/s. The properties of the reacting fluid are to be
considered identical to those of air at this temperature and pressure. The density of
the a -in.-catalyst particles is 120 lb/ft3 and the bed void fraction is 0.45. The rate law is
113 33
-r6, = kP, P, lb molflb cat. h
with3
lb mol
k = 0.0141 at 260°C
atm . Ib cat.. h
Ind. Eng. Chem., 45, 234 (1953).
- -- ~ - _I--
--
--