Page 351 - Elements of Chemical Reaction Engineering Ebook
P. 351
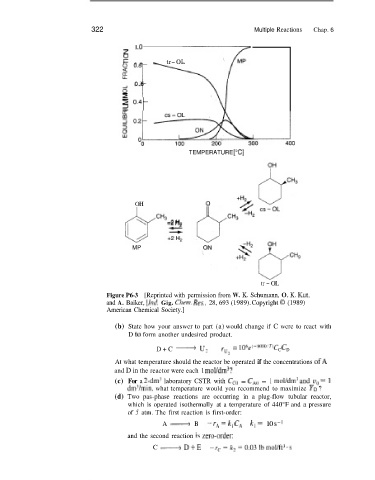
322 Multiple Reactions Chap. 6
1 .o
z
0
I- -- - tr-OL
0.6 u
2
2 0.41
2
TEMPERATURE ["C]
OH
3 " 3 2 +2 H,
MP
tr - OL
Figure P6-3 [Reprinted with permission from W. K. Schumann, 0. K. Kut,
and A. Baiker, [!d Gig. Chenz. RKS.. 28, 693 (1989). Copyright 0 (1989)
American Chemical Society.]
(b) State how your answer to part (a) would change if C were to react with
D to form another undesired product.
c
- IO~~(-~(I(X),~TIC
D+C U? vu1- C D
At what temperature should the reactor be operated if the concentrations ofA
and D in the reactor were each 1 mol/dm3?
(c) For a 2-dm3 laboratory CSTR with Ccc, = C,,, = I mol/dm3 and-u,, = 1
dm2/min, what temperature would you recommend to maximize YD '?
(d) Two pas-phase reactions are occurring in a plug-flow tubular reactor,
which is operated isothermally at a temperature of 440°F and a pressure
of 5 atm. The first reaction is first-order:
A + B -rA = k,C, k, = 10 s-'
and the second reaction i4 Lero-order:
C ---+ D+E -rc=k2=0.031bmol/ft3.s