Page 363 - Elements of Chemical Reaction Engineering Ebook
P. 363
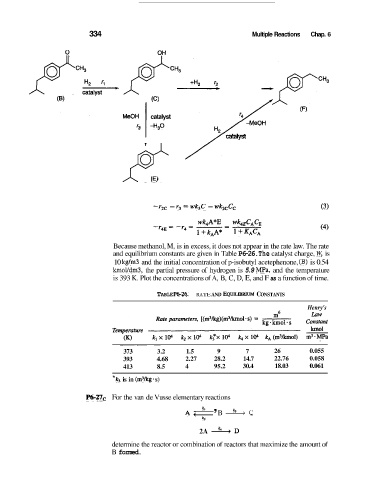
334 Multiple Reactions Chap. 6
MeOH catalyst
r3 -H,O catalyst -MeOH
P
(E)
-r2c = r, = wk3C = wk2pZc (3)
Because methanol, M, is in excess, it does not appear in the rate law. The rate
and equilibrium constants are given in Table P6-26. The catalyst charge, W, is
10 kgim3 and the initial concentration of p-isobutyl acetephenone, (B) is 0.54
kmoYdm3, the partial pressure of hydrogen is 5.9 MPa, and the temperature
is 393 K. Plot the concentrations of A, B, C, D, E, and F as a function of time.
TABLE ~6-26. RATE AND EQuILlBRIuM CONSTANTS
Henry's
~-
~
373 3.2 1.5 9 7 26 0.055
393 4.68 2.27 28.2 14.7 22.76 0.058
413 8.5 4 95.2 30.4 18.03 0.061
* k3 is in (m3kg s)
P6-27c For the van de Vusse elementary reactions
ki ,
B k3 ,C
k2
2A ' ,D
determine the reactor or combination of reactors that maximize the amount of
B formed.