Page 374 - Elements of Chemical Reaction Engineering Ebook
P. 374
Sec. 7.i! Searching for a Mechanism 345
Emmple 7-1 The Stern-Volmer Equation
Light is given off when a high-intensity ultrasonic wave is applied to water.5 This
light results from microsize bubbles being formed by the wave and then being com-
pressed by it. During the compression stage of the wave, the contents of the bubble
(e.g., water and whatever is dissolved in the water) are compressed adiabatically.
This compression gives rise to high temperatures, which generate active intemedi-
ates and cause chemical reactions to occur in the bubble. The intensity of the light
given off, I, is proportional to the rate of reaction of an activated water molecule
that has been formed in the microbubble.
H20* k3 > H20 + hv
intensity x (- rH ow) = k3 CHIo*
2
An order-of-magnitude increase in the intensity of sonoluminescence is
observed when either carbon disulfide or carbon tetrachloride is added to the water.
The intensity of luminescence, I, for the reaction
cs; k4 > cs, + hv
is
I OC (- res; ) = Wcs;
A siimilar result exists for CC14.
However, when an aliphatic alcohol, X, is added to the solution, the inten-
sity decreases with increasing concentration of alcohol. The data are usually
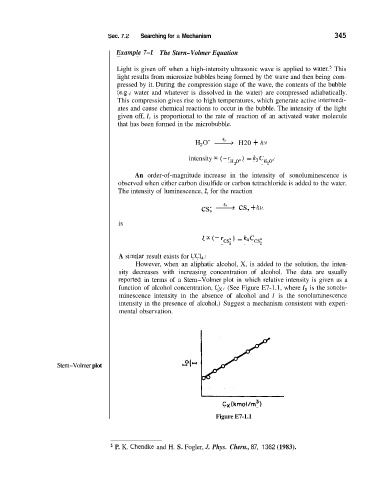
reported in terms of a Stern-Volmer plot in which relative intensity is given as a
function of alcohol concentration, C,. (See Figure E7-1.1, where I, is the sonolu-
minescence intensity in the absence of alcohol and I is the sonoluminesclence
intensity in the presence of alcohol.) Suggest a mechanism consistent with experi-
mental observation.
Stern-Volmer plot
Cx (kmol/rn3)
Figure E7-1.1
P. K. Chendke and H. S. Fogler, J. Phys. Chern., 87, 1362 (1983).