Page 379 - Elements of Chemical Reaction Engineering Ebook
P. 379
350 Nonelementary Reaction Kinetics Chap. 7
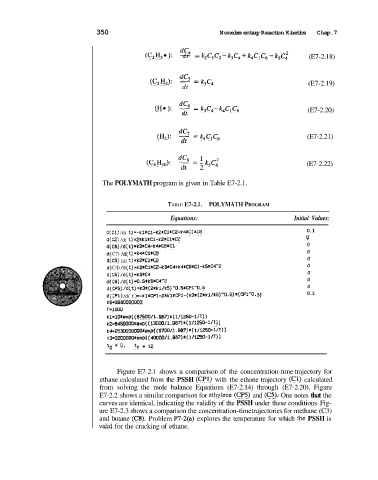
(C,H,*): 5 kzClC~-k3C4fk4C,C,-k,C~ (E7-2.18)
dt
=
dC5
(CZH4): - k3C4 (E7-2.19)
=
dt
dC6
(He): - k,C4-k4ClC, (E7-2.20)
dt
(Hz): 2 = k4C,C6 (E7-2.21)
dC8
(~4~10): - k.j~: (E7-2.22)
=
dt 2
The POLYMATH program is given in Table E7-2.1.
TABLE E7-2.1. POLYMATH PROGRAM
Equations: Initial Values:
d(C1) /d( t)~-kliC1-k2tCl*C2-C4*Cl~C6 0.1
d(C2) /d( t)=2*kl%Cl-K2*Cl*C2 0
d(C6)/d(t)~k3*C4-k4*CB*Cl 0
d( C7) /d( t) =k4*Cl*C6 0
d(C3) /d( t) =CZ*Cl*CZ 0
d( C4) /d( t) 0
d(CS)/d( t)=C3*C4 0
d(C8)/d( t)d0.5*k5*C4^2 0
d(CF'5) /d( t)=k3*( 2*kl/k5)*0.5XCPi^0.5 0
d( CPl) /d( t) =-ki*CP1-2*rl*CPl-(C3*(2*ltl/~~)"O.~)*(CF'~*O. 5) 0.1
C6=3960000000
T=iWO
Ll-lOxexp( (875Oo/l1 487) I( 1/1250-1/T))
1
~2=84~0~rexp((~30~/~.987)*(1/1250-~/T)
Cd-253ooMXl00xexp((9700/1.987)*( 1/125@1/T))
C3=320WOMe~p( (40000/l.Q87)*(l/l~O-l/T))
to - 0, tf = 12
Figure E7-2.1 shows a comparison of the concentration-time trajectory for
ethane calculated from the PSSH (CPI) with the ethane trajectory (Cl) calculated
from solving the mole balance Equations (E7-2.14) through (E7-2.20). Figure
E7-2.2 shows a similar comparison for ethyIene (CP5) and (C5). One notes that the
curves are identical, indicating the validity of the PSSH under these conditions. Fig-
ure E7-2.3 shows a comparison the concentration-time trajectories for methane (C3)
and butane (CS). Problem W-2(a) explores the temperature for which the PSSH is
valid for the cracking of ethane.