Page 381 - Elements of Chemical Reaction Engineering Ebook
P. 381
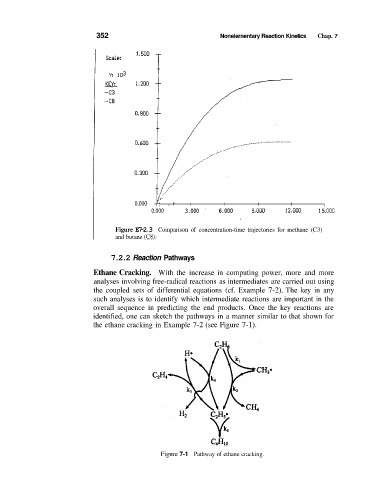
352 Nonelernentary Reaction Kinetics Chap. 7
I'
0.000 1 . : I I I I I
0.000 3.000 ' 6.000 9.000 1'2.000 3 5. OOC
1
Figure E7-2.3 Comparison of concentration-time trajectories for methane (C3)
and butane (C8).
7.2.2 Reaction Pathways
Ethane Cracking. With the increase in computing power, more and more
analyses involving free-radical reactions as intermediates are carried out using
the coupled sets of differential equations (cf. Example 7-2). The key in any
such analyses is to identify which intermediate reactions are important in the
overall sequence in predicting the end products. Once the key reactions are
identified, one can sketch the pathways in a manner similar to that shown for
the ethane cracking in Example 7-2 (see Figure 7-1).
Figure 7-1 Pathway of ethane cracking.