Page 396 - Elements of Chemical Reaction Engineering Ebook
P. 396
Sec. 7.3 Polymerization 367
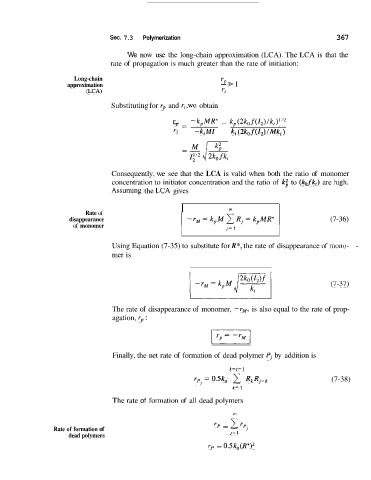
'We now use the long-chain approximation (LCA). The LCA is that the
rate of propagation is much greater than the rate of initiation:
Long-chain
approximation
(LCA)
Substituting for rp and ri,we obtain
-
rp
- = -k,MR" - kp(2k,f(12)/k,)1/2
Ti - ki MI ki (2 kof(Z,) / Mk, )
Consequently, we see that the LCA is valid when both the ratio of monomer
concentration to initiator concentration and the ratio of k: to (kofk,) are Ihigh.
Assunling the LCA gives
I I
Rate of
disappearance (7-36)
of monomer j= 1
Using Equation (7-35) to substitute for R*, the rate of disappearance of mono- -
mer is
(7-37)
The rate of disappearance of monomer, -rM, is also equal to the rate of prop-
agation, rp :
Finally, the net rate of formation of dead polymer PI by addition is
k=J-1
r5 = OSk, 2 RkRj-, (7-38)
k= 1
The rate of formation of all dead polymers
m
Rate of formation of rP = c YP,
dead polymers J= 1
rp = 0.5k,(R*)2