Page 404 - Elements of Chemical Reaction Engineering Ebook
P. 404
Sec. 7.3 Polymerization 375
M0L.W.
10,000 20,000 30,000 40,000 50,000 60,000
I I I I I I I
"
0 100 200 300 400 500
i
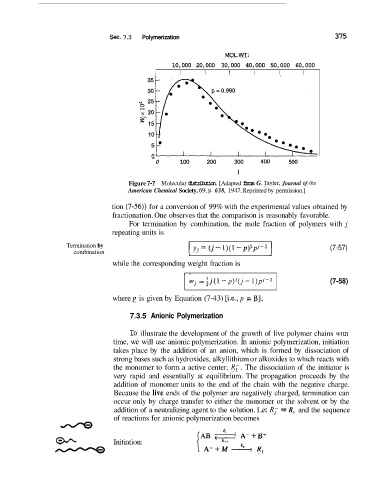
Figure 7-7 Molecular distribution. [Adapted from G. Tayler, Journal of rhe
American Chemical Society, 69, p. 638, 1947. Reprinted by permission.]
tion (7-56)] for a conversion of 99% with the experimental values obtained by
fractionation. One observes that the comparison is reasonably favorable.
For termination by combination, the mole fraction of polymers with j
repeating units is
Termination by 1 yj =; (j- 1)(1 -p)2pJ-2 I (7-57)
combination
L J
while the corresponding weight fraction is
I,
wj = ij(1 -p)3(j- 1)pJ-2 (7-58)
where p is given by Equation (7-43) [i.e., p = p].
7.3.5 Anionic Polymerization
To illustrate the development of the growth of live polymer chains witn
time, we will use anionic polymerization. In anionic polymerization, initiation
takes place by the addition of an anion, which is formed by dissociation of
strong bases such as hydroxides, alkyllithium or alkoxides to which reacts with
the monomer to form a active center, Ri. The dissociation of the initiator is
very rapid and essentially at equilibrium. The propagation proceeds by the
addition of monomer units to the end of the chain with the negative charge.
Because the live ends of the polymer are negatively charged, termination can
occur only by charge transfer to either the monomer or the solvent or by the
addition of a neutralizing agent to the solution. Let k; = R, and the sequence
of reactions for anionic polymerization becomes
d
OVL Initiation: A-fB+
R,