Page 41 - Elements of Chemical Reaction Engineering Ebook
P. 41
12 Mole Balances Chap. 1
-+I *Y I-
PFR Fi0 F,, exit
I *y, I 1Y+ AY
,
I I
I I
I I
.
Fj(Y) * Fj(Y+ AY)
-
-
@
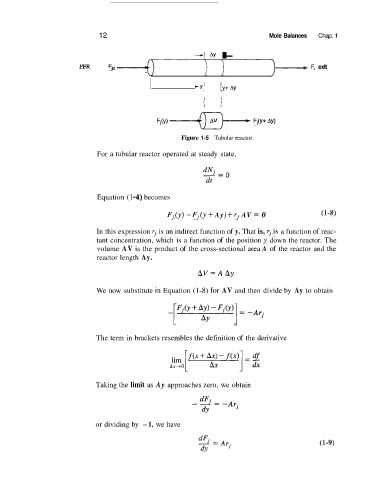
Figure 1-5 Tubular reactor.
For a tubular reactor operated at steady state,
Equation (1 -4) becomes
Fj,,(y) - Fj(y + Ay) + rj AV = 0 (1-8)
In this expression rj is an indirect function of y. That is, rj.is a function of reac-
tant concentration, which is a function of the position y down the reactor. The
volume AV is the product of the cross-sectional area A of the reactor and the
reactor length Ay.
hV=AAy
We now substitute in Equation (1-8) for AV and then divide by Ay to obtain
The term in brackets resembles the definition of the derivative
Taking the limit as Ay approaches zero, we obtain
or dividing by - 1, we have