Page 36 - Elements of Chemical Reaction Engineering Ebook
P. 36
Sec. 1.2 The General Mole Balance Equation 7
the cliemical species) are spatially uniform throughout the system volumle, the
rate of generation of species j, G,, is just the product of the reaction volume,
K and the rate of formation of species j, rJ.
moles
moles - - volume
-
time time * volume
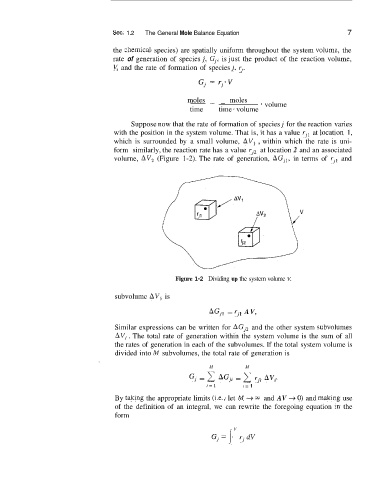
Suppose now that the rate of formation of species j for the reaction varies
with the position in the system volume. That is, it has a value rjl at locatiion 1,
which is surrounded by a small volume, AV, , within which the rate is uni-
form similarly, the reaction rate has a value rJz at location 2 and an associated
volurne, AV2 (Figure 1-2). The rate of generation, AG,,, in terms of rJ1 and
Figure 1-2 Dividing up the system volume V,
subvolume AV, is
AGJl = rJ1 AV,
Similar expressions can be written for AGJ2 and the other system subvollumes
AV, . The total rate of generation within the system volume is the sum of all
the rates of generation in each of the subvolumes. If the total system volume is
divided into M subvolumes, the total rate of generation is
M M
G~ = 1 AG~~ 2 rji ilvi,
=
1=1 I= 1
By tcalung the appropriate limits (Le., let M -+ 00 and AV -+ 0) and malung use
of the definition of an integral, we can rewrite the foregoing equation in the
form
V
rj dV