Page 31 - Elements of Chemical Reaction Engineering Ebook
P. 31
-
FL
2 Mole Balances Chap. 1 -_
J
reacting system. In this chapter we develop a general mole balance that can be
applied to any species (usually a chemical compound) entering, leaving, and/or
remaining within the reaction system volume. After defining the rate of reac-
tion, -rA, and discussing the earlier difficulties of properly defining the chem-
ical reaction rate, in this chapter we show how the general balance equation
may be used to develop a preliminary form of the design equations of the most
common industrial reactors: batch, continuous-stirred tank (CSTR), and tubu-
lar. In developing these equations, the assumptions pertaining to the modeling
of each type of reactor are delineated. Finally, a brief summary and series of
short review questions are given at the end of the chapter.
1.1 Definition of the Rate of Reaction, -rA
0
We begin our study by performing mole balances on each chemical species in
the system. Here, the term chemical species refers to any chemical compound
or element with a given identity. The identity of a chemical species is deter-
mined by the kind, number, and configuration of that species’ atoms. For
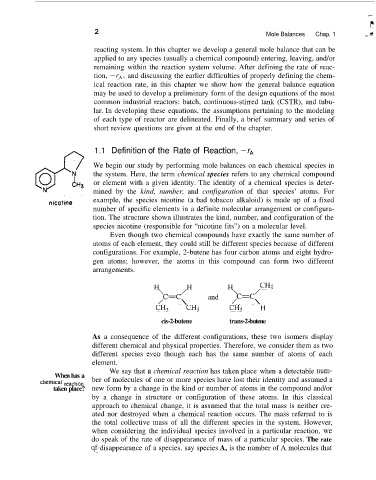
example, the species nicotine (a bad tobacco alkaloid) is made up of a fixed
number of specific elements in a definite molecular arrangement or configura-
tion. The structure shown illustrates the kind, number, and configuration of the
species nicotine (responsible for “nicotine fits”) on a molecular level.
Even though two chemical compounds have exactly the same number of
atoms of each element, they could still be different species because of different
configurations. For example, 2-butene has four carbon atoms and eight hydro-
gen atoms; however, the atoms in this compound can form two different
arrangements.
H H H CH3
\/
\/
/c=c and /C=C
\ \
CH3 CH3 CH:, H
cis-2-butene trans-2- butene
As a consequence of the different configurations, these two isomers display
different chemical and physical properties. Therefore, we consider them as two
different species evw though each has the same number of atoms of each
element.
We say that a chemical reaction has taken place when a detectable num-
When has a
reaction ber of molecules of one or more species have lost their identity and assumed a
taken place? new form by a change in the kind or number of atoms in the compound and/or
by a change in structure or configuration of these atoms. In this classical
approach to chemical change, it is assumed that the total mass is neither cre-
ated nor destroyed when a chemical reaction occurs. The mass referred to is
the total collective mass of all the different species in the system. However,
when considering the individual species involved in a particular reaction, we
do speak of the rate of disappearance of mass of a particular species. The rate
9f disappearance of a species, say species A, is the number of A molecules that