Page 63 - Elements of Chemical Reaction Engineering Ebook
P. 63
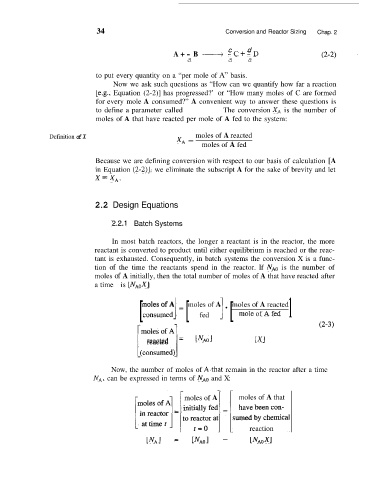
34 Conversion and Reactor Sizing Chap. 2
b c d
A+ - B ___j -C+-D
a a a
to put every quantity on a “per mole of A” basis.
Now we ask such questions as “How can we quantify how far a reaction
[e.g., Equation (2-2)] has progressed?’ or “How many moles of C are formed
for every mole A consumed?” A convenient way to answer these questions is
to define a parameter called conversion. The conversion XA is the number of
moles of A that have reacted per mole of A fed to the system:
Definition of X moles of A reacted
XA =
moles of A fed
Because we are defining conversion with respect to our basis of calculation [A
in Equation (2-2)], we eliminate the subscript A for the sake of brevity and let
X=X*.
2.2 Design Equations
‘2.2.1 Batch Systems
In most batch reactors, the longer a reactant is in the reactor, the more
reactant is converted to product until either equilibrium is reached or the reac-
tant is exhausted. Consequently, in batch systems the conversion X is a func-
tion of the time the reactants spend in the reactor. If NAO is the number of
moles of A initially, then the total number of moles of A that have reacted after
a time t is [NAOX]
[ consumedl = [ fed ] [ mole of A fed 1
moles of A
moles of A
moles of A reacted
1
reacted = [ N ~ ~ [XI
[(consumedl
Now, the number of moles of A4hat remain in the reactor after a time t,
NA, can be expressed in terms of NAD and X:
moles of A moles of A that
reaction
[NA] = [NAO] - [NAOxl