Page 188 - Encyclopedia of Chemical Compounds 3 Vols
P. 188
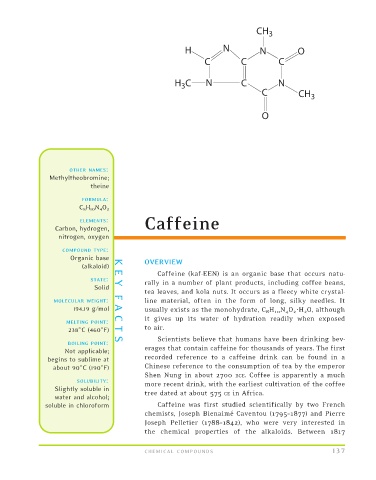
CH 3
H N N O
C C C
H C N C N
3
C CH 3
O
OTHER NAMES:
Methyltheobromine;
theine
FORMULA:
C 8 H 10 N 4 O 2
ELEMENTS: Caffeine
Carbon, hydrogen,
nitrogen, oxygen
COMPOUND TYPE:
OVERVIEW
(alkaloid)
Caffeine (kaf-EEN) is an organic base that occurs natu-
Organic base KE
STATE:
rally in a number of plant products, including coffee beans,
Solid
Y
tea leaves, and kola nuts. It occurs as a fleecy white crystal-
MOLECULAR WEIGHT: F line material, often in the form of long, silky needles. It
194.19 g/mol A usually exists as the monohydrate, C 8 H 10 N 4 O 2 H 2 O, although
it gives up its water of hydration readily when exposed
MELTING POINT:
C
to air.
238 C (460 F) T
Scientists believe that humans have been drinking bev-
BOILING POINT:
S
erages that contain caffeine for thousands of years. The first
Not applicable;
recorded reference to a caffeine drink can be found in a
begins to sublime at
about 90 C (190 F) Chinese reference to the consumption of tea by the emperor
Shen Nung in about 2700 BCE. Coffee is apparently a much
SOLUBILITY:
more recent drink, with the earliest cultivation of the coffee
Slightly soluble in
tree dated at about 575 CE in Africa.
water and alcohol;
soluble in chloroform Caffeine was first studied scientifically by two French
chemists, Joseph Bienaime ´ Caventou (1795–1877) and Pierre
Joseph Pelletier (1788–1842), who were very interested in
the chemical properties of the alkaloids. Between 1817
CHEMICAL COMPOUNDS 137