Page 241 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 241
P1: FYK/GJK P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN005B-205 June 15, 2001 20:24
148 Electrochemical Engineering
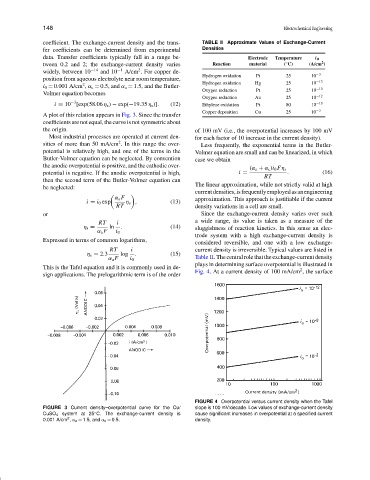
coefficient. The exchange-current density and the trans- TABLE II Approximate Values of Exchange-Current
fer coefficients can be determined from experimental Densities
data. Transfer coefficients typically fall in a range be- Electrode Temperature i 0
2
◦
tween 0.2 and 2; the exchange-current density varies Reaction material ( C) (A/cm )
2
widely, between 10 −14 and 10 −1 A/cm . For copper de-
Hydrogen oxidation Pt 25 10 −3
position from aqueous electrolyte near room temperature,
Hydrogen oxidation Hg 25 10 −13
2
i 0 = 0.001 A/cm , α c = 0.5, and α a = 1.5, and the Butler-
Oxygen reduction Pt 25 10 −10
Volmer equation becomes
Oxygen reduction Au 25 10 −12
−3
i = 10 [exp(58.06 η s ) − exp(−19.35 η s )]. (12) Ethylene oxidation Pt 80 10 −10
Copper deposition Cu 25 10 −3
A plot of this relation appears in Fig. 3. Since the transfer
coefficients are not equal, the curve is not symmetric about
the origin. of 100 mV (i.e., the overpotential increases by 100 mV
Most industrial processes are operated at current den- for each factor of 10 increase in the current density).
2
sities of more than 50 mA/cm . In this range the over- Less frequently, the exponential terms in the Butler-
potential is relatively high, and one of the terms in the Volmer equation are small and can be linearized, in which
Butler-Volmer equation can be neglected. By convention case we obtain
the anodic overpotential is positive, and the cathodic over-
(α a + α c )i 0 Fη s
potential is negative. If the anodic overpotential is high, i = . (16)
RT
then the second term of the Butler-Volmer equation can
The linear approximation, while not strictly valid at high
be neglected:
currentdensities,isfrequentlyemployedasanengineering
α a F approximation. This approach is justifiable if the current
i = i 0 exp η s , (13)
RT density variations in a cell are small.
or Since the exchange-current density varies over such
RT i a wide range, its value is taken as a measure of the
η s = ln . (14) sluggishness of reaction kinetics. In this sense an elec-
α a F i 0
trode system with a high exchange-current density is
Expressed in terms of common logarithms,
considered reversible, and one with a low exchange-
RT i current density is irreversible. Typical values are listed in
η s = 2.3 log . (15)
α a F i 0 Table II. The central role that the exchange-current density
plays in determining surface overpotential is illustrated in
This is the Tafel equation and it is commonly used in de-
2
Fig. 4. At a current density of 100 mA/cm , the surface
sign applications. The prelogarithmic term is of the order
FIGURE 4 Overpotential versus current density when the Tafel
FIGURE 3 Current density–overpotential curve for the Cu/ slope is 100 mV/decade. Low values of exchange-current density
◦
CuSO 4 system at 25 C. The exchange-current density is cause significant increases in overpotential at a specified current
2
0.001 A/cm , α a = 1.5, and α c = 0.5. density.