Page 63 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 63
P1: FWD Revised Pages
Encyclopedia of Physical Science and Technology En001c-14 May 7, 2001 18:25
286 Aerosols
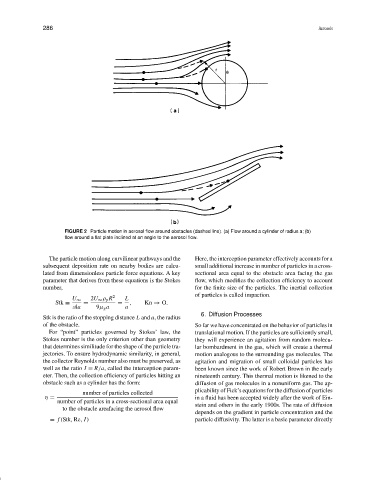
FIGURE 2 Particle motion in aerosol flow around obstacles (dashed line). (a) Flow around a cylinder of radius a; (b)
flow around a flat plate inclined at an angle to the aerosol flow.
The particle motion along curvilinear pathways and the Here, the interception parameter effectively accounts for a
subsequent deposition rate on nearby bodies are calcu- small additional increase in number of particles in a cross-
lated from dimensionless particle force equations. A key sectional area equal to the obstacle area facing the gas
parameter that derives from these equations is the Stokes flow, which modifies the collection efficiency to account
number, for the finite size of the particles. The inertial collection
of particles is called impaction.
2
2U ∞ ρ p R L
U ∞
Stk ≡ = = , Kn → O.
a 9µ g a a
6. Diffusion Processes
Stk is the ratio of the stopping distance L and a, the radius
of the obstacle. So far we have concentrated on the behavior of particles in
For “point” particles governed by Stokes’ law, the translational motion. If the particles are sufficiently small,
Stokes number is the only criterion other than geometry they will experience an agitation from random molecu-
that determines similitude for the shape of the particle tra- lar bombardment in the gas, which will create a thermal
jectories. To ensure hydrodynamic similarity, in general, motion analogous to the surrounding gas molecules. The
the collector Reynolds number also must be preserved, as agitation and migration of small colloidal particles has
well as the ratio I ≡ R/a, called the interception param- been known since the work of Robert Brown in the early
eter. Then, the collection efficiency of particles hitting an nineteenth century. This thermal motion is likened to the
obstacle such as a cylinder has the form: diffusion of gas molecules in a nonuniform gas. The ap-
plicability of Fick’s equations for the diffusion of particles
number of particles collected
η = in a fluid has been accepted widely after the work of Ein-
number of particles in a cross-sectional area equal
stein and others in the early 1900s. The rate of diffusion
to the obstacle areafacing the aerosol flow
depends on the gradient in particle concentration and the
= f (Stk, Re, I) particle diffusivity. The latter is a basic parameter directly