Page 112 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 112
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
460 Catalysis, Homogeneous
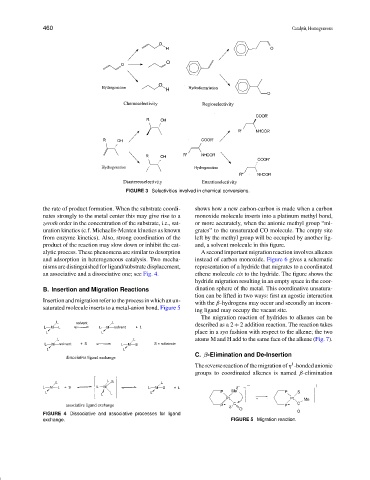
FIGURE 3 Selectivities involved in chemical conversions.
the rate of product formation. When the substrate coordi- shows how a new carbon-carbon is made when a carbon
nates strongly to the metal center this may give rise to a monoxide molecule inserts into a platinum methyl bond,
zeroth order in the concentration of the substrate, i.e., sat- or more accurately, when the anionic methyl group “mi-
uration kinetics (c.f. Michaelis-Menten kinetics as known grates” to the unsaturated CO molecule. The empty site
from enzyme kinetics). Also, strong coordination of the left by the methyl group will be occupied by another lig-
product of the reaction may slow down or inhibit the cat- and, a solvent molecule in this figure.
alytic process. These phenomena are similar to desorption A second important migration reaction involves alkenes
and adsorption in heterogeneous catalysis. Two mecha- instead of carbon monoxide. Figure 6 gives a schematic
nisms are distinguished for ligand/substrate displacement, representation of a hydride that migrates to a coordinated
an associative and a dissociative one; see Fig. 4. ethene molecule cis to the hydride. The figure shows the
hydride migration resulting in an empty space in the coor-
B. Insertion and Migration Reactions dination sphere of the metal. This coordinative unsatura-
tion can be lifted in two ways: first an agostic interaction
Insertion and migration refer to the process in which an un-
with the β-hydrogens may occur and secondly an incom-
saturated molecule inserts to a metal-anion bond. Figure 5
ing ligand may occupy the vacant site.
The migration reaction of hydrides to alkenes can be
described as a 2 + 2 addition reaction. The reaction takes
place in a syn fashion with respect to the alkene; the two
atoms M and H add to the same face of the alkene (Fig. 7).
C. β-Elimination and De-Insertion
1
The reverse reaction of the migration of η -bonded anionic
groups to coordinated alkenes is named β-elimination
FIGURE 4 Dissociative and associative processes for ligand
exchange. FIGURE 5 Migration reaction.