Page 161 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 161
P1: GTY/GWT P2: GLM Final
Encyclopedia of Physical Science and Technology EN006K-933 June 29, 2001 12:14
Fuel Chemistry 271
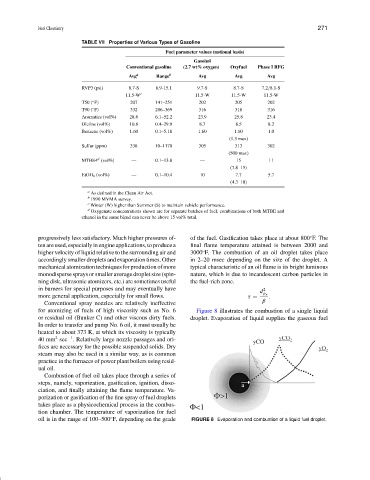
TABLE VII Properties of Various Types of Gasoline
Fuel parameter values (national basis)
Gasohol
Conventional gasoline (2.7 wt% oxygen) Oxyfuel Phase I RFG
Avg a Range b Avg Avg Avg
RVP3 (psi) 8.7-S 6.9-15.1 9.7-S 8.7-S 7.2/8.1-S
11.5-W c 11.5-W 11.5-W 11.5-W
◦
T50 ( F) 207 141–251 202 205 202
T90 ( F) 332 286–369 316 318 316
◦
Aromatics (vol%) 28.6 6.1–52.2 23.9 25.8 23.4
Olefins (vol%) 10.8 0.4–29.9 8.7 8.5 8.2
Benzene (vol%) 1.60 0.1–5.18 1.60 1.60 1.0
(1.3 max)
Sulfur (ppm) 338 10–1170 305 313 302
(500 max)
d
MTBE4 (vol%) — 0.1–13.8 — 15 11
(7.8–15)
EtOH 4 (vol%) — 0.1–10.4 10 7.7 5.7
(4.3–10)
a As defined in the Clean Air Act.
b 1990 MVMA survey.
c Winter (W) higher than Summer (S) to maintain vehicle performance.
d Oxygenate concentrations shown are for separate batches of fuel; combinations of both MTBE and
ethanol in the same blend can never be above 15 vol% total.
progressively less satisfactory. Much higher pressures of- of the fuel. Gasification takes place at about 800 F. The
◦
tenareused,especiallyinengineapplications,toproducea final flame temperature attained is between 2000 and
◦
higher velocity of liquid relative to the surrounding air and 3000 F. The combustion of an oil droplet takes place
accordingly smaller droplets and evaporation times. Other in 2–20 msec depending on the size of the droplet. A
mechanicalatomizationtechniquesforproductionofmore typical characteristic of an oil flame is its bright luminous
monodisperse sprays or smaller average droplet size (spin- nature, which is due to incandescent carbon particles in
ning disk, ultrasonic atomizers, etc.) are sometimes useful the fuel-rich zone.
in burners for special purposes and may eventually have 2
d
more general application, especially for small flows. τ = p p .
Conventional spray nozzles are relatively ineffective β
for atomizing of fuels of high viscosity such as No. 6 Figure 8 illustrates the combustion of a single liquid
or residual oil (Bunker C) and other viscous dirty fuels. droplet. Evaporation of liquid supplies the gaseous fuel
In order to transfer and pump No. 6 oil, it must usually be
heated to about 373 K, at which its viscosity is typically
2
−1
40 mm sec . Relatively large nozzle passages and ori-
fices are necessary for the possible suspended solids. Dry
steam may also be used in a similar way, as is common
practice in the furnaces of power plant boilers using resid-
ual oil.
Combustion of fuel oil takes place through a series of
steps, namely, vaporization, gasification, ignition, disso-
ciation, and finally attaining the flame temperature. Va-
porization or gasification of the fine spray of fuel droplets
takes place as a physicochemical process in the combus-
tion chamber. The temperature of vaporization for fuel
◦
oil is in the range of 100–500 F, depending on the grade FIGURE 8 Evaporation and combustion of a liquid fuel droplet.