Page 192 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 192
P1: LLL/GVX P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN011G-539 July 14, 2001 21:48
Organic Chemical Systems, Theory 439
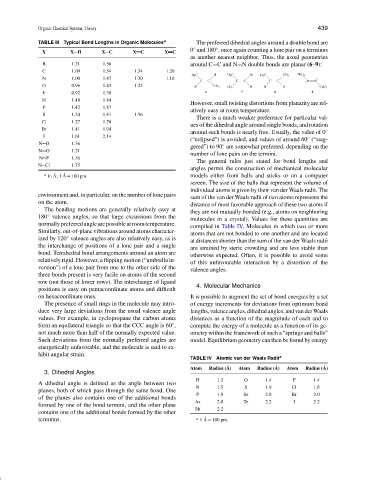
TABLE III Typical Bond Lengths in Organic Molecules a The preferred dihedral angles around a double bond are
0 and 180 , once again counting a lone pair on a terminus
◦
◦
X X H X C X C X C
as another nearest neighbor. Thus, the usual geometries
B 1.21 1.56 around C=C and N=N double bonds are planar (6–9):
C 1.09 1.54 1.34 1.20
H 3 C H H 3 C H H 3 C CH 3 H 5 C 6
N 1.00 1.47 1.30 1.16
C C C C C C N N
O 0.96 1.43 1.22 H CH 3 H 3 C H H H C 6 H 5
F 0.92 1.38 6 7 8 9
Si 1.48 1.84
However, small twisting distortions from planarity are rel-
P 1.42 1.87
atively easy at room temperature.
S 1.34 1.81 1.56
There is a much weaker preference for particular val-
Cl 1.27 1.76
ues of the dihedral angle around single bonds, and rotation
Br 1.41 1.94
around such bonds is nearly free. Usually, the value of 0 ◦
I 1.61 2.14
◦
(“eclipsed”) is avoided, and values of around 60 (“stag-
N O 1.36
◦
gered”)to90 are somewhat preferred, depending on the
N O 1.21
number of lone pairs on the termini.
N F 1.36
The general rules just stated for bond lengths and
N Cl 1.75
angles permit the construction of mechanical molecular
a
In ˚ A; 1 ˚ A = 100 pm. models either from balls and sticks or on a computer
screen. The size of the balls that represent the volume of
individual atoms is given by their van der Waals radii. The
environment and, in particular, on the number of lone pairs
sum of the van der Waals radii of two atoms represents the
on the atom.
distance of most favorable approach of these two atoms if
The bending motions are generally relatively easy at they are not mutually bonded (e.g., atoms on neighboring
◦
180 valence angles, so that large excursions from the molecules in a crystal). Values for these quantities are
normallypreferredanglearepossibleatroomtemperature. compiled in Table IV. Molecules in which two or more
Similarly, out-of-plane vibrations around atoms character-
atoms that are not bonded to one another and are located
ized by 120 valence angles are also relatively easy, as is
◦
at distances shorter than the sum of the van der Waals radii
the interchange of positions of a lone pair and a single
are strained by steric crowding and are less stable than
bond. Tetrahedral bond arrangements around an atom are
otherwise expected. Often, it is possible to avoid some
relatively rigid. However, a flipping motion (“umbrella in-
of this unfavourable interaction by a distortion of the
version”) of a lone pair from one to the other side of the
valence angles.
three bonds present is very facile on atoms of the second
row (not those of lower rows). The interchange of ligand
4. Molecular Mechanics
positions is easy on pentacoordinate atoms and difficult
on hexacoordinate ones. It is possible to augment the set of bond energies by a set
The presence of small rings in the molecule may intro- of energy increments for deviations from optimum bond
duce very large deviations from the usual valence angle lengths,valenceangles,dihedralangles,andvanderWaals
values. For example, in cyclopropane the carbon atoms distances as a function of the magnitude of each and to
◦
form an equilateral triangle so that the CCC angle is 60 , compute the energy of a molecule as a function of its ge-
not much more than half of the normally expected value. ometry within the framework of such a “springs and balls”
Such deviations from the normally preferred angles are model. Equilibrium geometry can then be found by energy
energetically unfavorable, and the molecule is said to ex-
hibit angular strain.
TABLE IV Atomic van der Waals Radii a
Atom Radius ( ˚ A) Atom Radius ( ˚ A) Atom Radius ( ˚ A)
3. Dihedral Angles
H 1.2 O 1.4 F 1.4
A dihedral angle is defined as the angle between two
N 1.5 S 1.9 Cl 1.8
planes, both of which pass through the same bond. One
P 1.9 Se 2.0 Br 2.0
of the planes also contains one of the additional bonds
As 2.0 Te 2.2 I 2.2
formed by one of the bond termini, and the other plane
Sb 2.2
contains one of the additional bonds formed by the other
terminus. a 1 ˚ A = 100 pm.