Page 233 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 233
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN011A-543 February 12, 2002 12:40
520 Organic Macrocycles
TABLE I Radii of Cations and of Crown Ether
Cavities
Cation Radius ( ˚ A)
Li + 0.74
Na + 1.02
K + 1.38
Rb + 1.49
Cs + 1.70
Mg 2+ 0.72
Ca 2+ 1.00
Sr 2+ 1.16
Ba 2+ 1.36
Ag + 1.15
Tl + 1.50
Pb 2+ 1.18
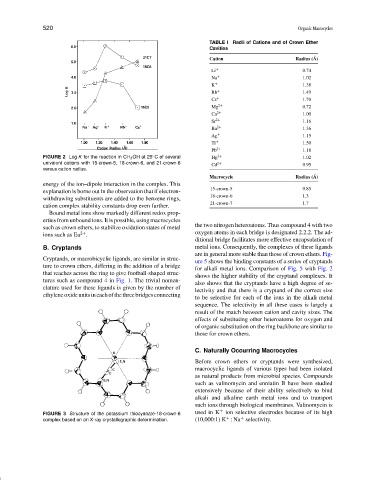
FIGURE 2 Log K for the reaction in CH 3 OH at 25 C of several Hg 2+ 1.02
◦
univalent cations with 15-crown-5, 18-crown-6, and 21-crown-6
Cd 2+ 0.95
versus cation radius.
Macrocycle Radius ( ˚ A)
energy of the ion–dipole interaction in the complex. This
15-crown-5 0.85
explanation is borne out in the observation that if electron-
18-crown-6 1.3
withdrawing substituents are added to the benzene rings,
21-crown-7 1.7
cation complex stability constants drop even further.
Bound metal ions show markedly different redox prop-
erties from unbound ions. It is possible, using macrocycles
the two nitrogen heteroatoms. Thus compound 4 with two
such as crown ethers, to stabilize oxidation states of metal
oxygen atoms in each bridge is designated 2.2.2. The ad-
ions such as Eu .
2+
ditional bridge facilitates more effective encapsulation of
B. Cryptands metal ions. Consequently, the complexes of these ligands
are in general more stable than those of crown ethers. Fig-
Cryptands, or macrobicyclic ligands, are similar in struc-
ure 5 shows the binding constants of a series of cryptands
ture to crown ethers, differing in the addition of a bridge
for alkali metal ions. Comparison of Fig. 5 with Fig. 2
that reaches across the ring to give football-shaped struc-
shows the higher stability of the cryptand complexes. It
tures such as compound 4 in Fig. 1. The trivial nomen-
also shows that the cryptands have a high degree of se-
clature used for these ligands is given by the number of
lectivity and that there is a cryptand of the correct size
ethyleneoxideunitsineachofthethreebridgesconnecting
to be selective for each of the ions in the alkali metal
sequence. The selectivity in all these cases is largely a
result of the match between cation and cavity sizes. The
effects of substituting other heteroatoms for oxygen and
of organic substitution on the ring backbone are similar to
those for crown ethers.
C. Naturally Occurring Macrocycles
Before crown ethers or cryptands were synthesized,
macrocyclic ligands of various types had been isolated
as natural products from microbial species. Compounds
such as valinomycin and enniatin B have been studied
extensively because of their ability selectively to bind
alkali and alkaline earth metal ions and to transport
such ions through biological membranes. Valinomycin is
+
FIGURE 3 Structure of the potassium thiocyanate-18-crown-6 used in K ion selective electrodes because of its high
+
+
complex based on an X-ray crystallographic determination. (10,000:1) K :Na selectivity.