Page 238 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 238
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN011A-543 February 12, 2002 12:40
Organic Macrocycles 525
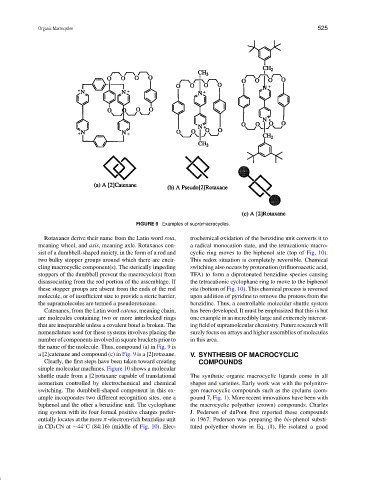
FIGURE 9 Examples of supramacrocycles.
Rotaxanes derive their name from the Latin word rota, trochemical oxidation of the benzidine unit converts it to
meaning wheel, and axis, meaning axle. Rotaxanes con- a radical monocation state, and the tetracationic macro-
sist of a dumbbell-shaped moiety, in the form of a rod and cyclic ring moves to the biphenol site (top of Fig. 10).
two bulky stopper groups around which there are encir- This redox situation is completely reversible. Chemical
cling macrocyclic component(s). The sterically impeding switching also occurs by protonation (trifluoroacetic acid,
stoppers of the dumbbell prevent the macrocycle(s) from TFA) to form a diprotonated benzidine species causing
disassociating from the rod portion of the assemblage. If the tetracationic cyclophane ring to move to the biphenol
these stopper groups are absent from the ends of the rod site (bottom of Fig. 10). This chemical process is reversed
molecule, or of insufficient size to provide a steric barrier, upon addition of pyridine to remove the protons from the
the supramolecules are termed a pseudorotaxane. benzidine. Thus, a controllable molecular shuttle system
Catenanes, from the Latin word catena, meaning chain, has been developed. It must be emphasized that this is but
are molecules containing two or more interlocked rings one example in an incredibly large and extremely interest-
that are inseparable unless a covalent bond is broken. The ing field of supramolecular chemistry. Future research will
nomenclature used for these systems involves placing the surely focus on arrays and higher assemblies of molecules
number of components involved in square brackets prior to in this area.
the name of the molecule. Thus, compound (a) in Fig. 9 is
a [2]catenane and compound (c) in Fig. 9 is a [2]rotaxane. V. SYNTHESIS OF MACROCYCLIC
Clearly, the first steps have been taken toward creating COMPOUNDS
simple molecular machines. Figure 10 shows a molecular
shuttle made from a [2]rotaxane capable of translational The synthetic organic macrocyclic ligands come in all
isomerism controlled by electrochemical and chemical shapes and varieties. Early work was with the polynitro-
switching. The dumbbell-shaped component in this ex- gen macrocyclic compounds such as the cyclams (com-
ample incorporates two different recognition sites, one a pound 7, Fig. 1). More recent innovations have been with
biphenol and the other a benzidine unit. The cyclophane the macrocyclic polyether (crown) compounds. Charles
ring system with its four formal positive charges prefer- J. Pedersen of duPont first reported these compounds
entially locates at the more π-electron-rich benzidine unit in 1967. Pedersen was preparing the bis-phenol substi-
in CD 3 CN at −44 C (84:16) (middle of Fig. 10). Elec- tuted polyether shown in Eq. (1). He isolated a good
◦