Page 239 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 239
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN011A-543 February 12, 2002 12:40
526 Organic Macrocycles
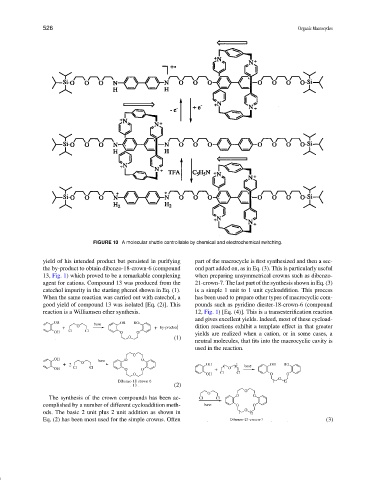
FIGURE 10 A molecular shuttle controllable by chemical and electrochemical switching.
yield of his intended product but persisted in purifying part of the macrocycle is first synthesized and then a sec-
the by-product to obtain dibenzo-18-crown-6 (compound ond part added on, as in Eq. (3). This is particularly useful
13, Fig. 1) which proved to be a remarkable complexing when preparing unsymmetrical crowns such as dibenzo-
agent for cations. Compound 13 was produced from the 21-crown-7. The last part of the synthesis shown in Eq. (3)
catechol impurity in the starting phenol shown in Eq. (1). is a simple 1 unit to 1 unit cycloaddition. This process
When the same reaction was carried out with catechol, a has been used to prepare other types of macrocyclic com-
good yield of compound 13 was isolated [Eq. (2)]. This pounds such as pyridino diester-18-crown-6 (compound
reaction is a Williamsen ether synthesis. 12, Fig. 1) [Eq. (4)]. This is a transesterification reaction
and gives excellent yields. Indeed, most of these cycload-
dition reactions exhibit a template effect in that greater
yields are realized when a cation, or in some cases, a
(1)
neutral molecules, that fits into the macrocyclic cavity is
used in the reaction.
(2)
The synthesis of the crown compounds has been ac-
complished by a number of different cycloaddition meth-
ods. The basic 2 unit plus 2 unit addition as shown in
Eq. (2) has been most used for the simple crowns. Often (3)