Page 24 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 24
P1: GLQ Revised Pages
Encyclopedia of Physical Science and Technology EN001F-4 May 7, 2001 16:19
76 Acetylene
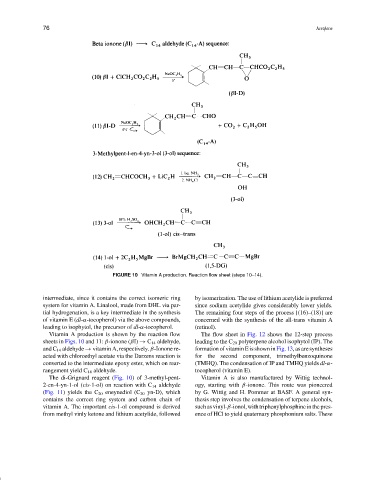
FIGURE 10 Vitamin A production. Reaction flow sheet (steps 10–14).
intermediate, since it contains the correct isomeric ring by isomerization. The use of lithium acetylide is preferred
system for vitamin A. Linalool, made from DHL via par- since sodium acetylide gives considerably lower yields.
tial hydrogenation, is a key intermediate in the synthesis The remaining four steps of the process [(16)–(18)] are
of vitamin E (dl-α-tocopherol) via the above compounds, concerned with the synthesis of the all-trans vitamin A
leading to isophytol, the precursor of dl-α-tocopherol. (retinol).
Vitamin A production is shown by the reaction flow The flow sheet in Fig. 12 shows the 12-step process
sheets in Figs. 10 and 11: β-ionone (βI) → C 14 aldehyde, leading to the C 20 polyterpene alcohol isophytol (IP). The
and C 14 aldehyde → vitamin A, respectively. β-Ionone re- formationofvitaminEisshowninFig.13,asaresyntheses
acted with chloroethyl acetate via the Darzens reaction is for the second component, trimethylbenzoquinone
converted to the intermediate epoxy ester, which on rear- (TMHQ). The condensation of IP and TMHQ yields dl-α-
rangement yield C 14 aldehyde. tocopherol (vitamin E).
The di-Grignard reagent (Fig. 10) of 3-methyl-pent- Vitamin A is also manufactured by Wittig technol-
2-en-4-yn-1-ol (cis-1-ol) on reaction with C 14 aldehyde ogy, starting with β-ionone. This route was pioneered
(Fig. 11) yields the C 20 eneynediol (C 20 yn-D), which by G. Wittig and H. Pommer at BASF. A general syn-
contains the correct ring system and carbon chain of thesis step involves the condensation of terpene alcohols,
vitamin A. The important cis-1-ol compound is derived suchasvinyl-β-ionol,withtriphenylphosphineinthepres-
from methyl vinly ketone and lithium acetylide, followed ence of HCl to yield quaternary phosphonium salts. These