Page 84 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 84
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
392 Carbohydrates
though the terminal (primary) hydroxyl groups of sugars
can also undergo oxidation by these reagents, the reaction
occurs at a somewhat lower rate, so that the oxidation can
be stopped at the monocarboxylic acid (or lactone) stage.
By the use of moderately vigorous conditions, oxidations
of the primary hydroxyl groups have been used to convert
glycosides to glycosiduronic acids. The oxidation of sec-
ondary hydroxyl groups to keto groups requires drastic
conditions, and these may be accompanied by cleavage
of C C bonds. A study of the above-mentioned oxida-
tions has revealed that changes in the reaction conditions
(temperature, acidity, and concentration) are often accom-
panied by changes in the nature of the oxidizing species.
For example, the concentration of hypohalous acid is very
low, but when sufficient alkali is added to the system, the
concentration of hypohalite ion increases dramatically (at
pH 1, 82% of the total chlorine exists as free chlorine
and 18% as hypochlorous acid; at pH 4, only 0.4% of the
chlorine is free and 99.6% exists as hypochlorous acid;
and at pH 8, 21% exists as hypochlorous acid and 79%
as hypochlorite). The order of effectiveness of halogens
as oxidants is Br 2 > Cl 2 > I 2 , the last being quite ineffec-
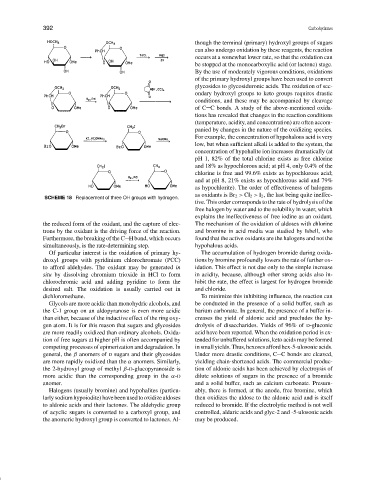
SCHEME 18 Replacement of three OH groups with hydrogen.
tive. This order corresponds to the rate of hydrolysis of the
free halogen by water and to the solubility in water, which
explains the ineffectiveness of free iodine as an oxidant.
the reduced form of the oxidant, and the capture of elec- The mechanism of the oxidation of aldoses with chlorine
trons by the oxidant is the driving force of the reaction. and bromine in acid media was studied by Isbell, who
Furthermore, the breaking of the C H bond, which occurs found that the active oxidants are the halogens and not the
simultaneously, is the rate-determining step. hypohalous acids.
Of particular interest is the oxidation of primary hy- The accumulation of hydrogen bromide during oxida-
droxyl groups with pyridinium chlorochromate (PCC) tions by bromine profoundly lowers the rate of further ox-
to afford aldehydes. The oxidant may be generated in idation. This effect is not due only to the simple increase
situ by dissolving chromium trioxide in HCl to form in acidity, because, although other strong acids also in-
chlorochromic acid and adding pyridine to form the hibit the rate, the effect is largest for hydrogen bromide
desired salt. The oxidation is usually carried out in and chloride.
dichloromethane. To minimize this inhibiting influence, the reaction can
Glycols are more acidic than monohydric alcohols, and be conducted in the presence of a solid buffer, such as
the C-1 group on an aldopyranose is even more acidic barium carbonate. In general, the presence of a buffer in-
than either, because of the inductive effect of the ring oxy- creases the yield of aldonic acid and precludes the hy-
gen atom. It is for this reason that sugars and glycosides drolysis of disaccharides. Yields of 96% of D-gluconic
are more readily oxidized than ordinary alcohols. Oxida- acid have been reported. When the oxidation period is ex-
tion of free sugars at higher pH is often accompanied by tended for unbuffered solutions, keto acids may be formed
competing processes of epimerization and degradation. In in small yields. Thus, hexoses afford hex-5-ulosonic acids.
general, the β anomers of D sugars and their glycosides Under more drastic conditions, C C bonds are cleaved,
are more rapidly oxidized than the α anomers. Similarly, yielding chain-shortened acids. The commercial produc-
the 2-hydroxyl group of methyl β-D-glucopyranoside is tion of aldonic acids has been achieved by electroysis of
more acidic than the corresponding group in the α-D dilute solutions of sugars in the presence of a bromide
anomer. and a solid buffer, such as calcium carbonate. Presum-
Halogens (usually bromine) and hypohalites (particu- ably, there is formed, at the anode, free bromine, which
larlysodiumhypoiodite)havebeenusedtooxidizealdoses then oxidizes the aldose to the aldonic acid and is itself
to aldonic acids and their lactones. The aldehydic group reduced to bromide. If the electrolytic method is not well
of acyclic sugars is converted to a carboxyl group, and controlled, aldaric acids and glyc-2 and -5-ulosonic acids
the anomeric hydroxyl group is converted to lactones. Al- may be produced.