Page 81 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 81
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
Carbohydrates 389
SCHEME 14 Formation of 2,3-O-ispropylidene rings.
The foregoing generalizations are useful when one
is planning multistage syntheses of complex sugar
molecules, especially when it is advantageous to block
certain hydroxyl groups selectively and leave the other
ones free for subsequent reaction. They are also useful in SCHEME 15 Formation of penta-O-acetyl-D-glucoyranose and
furanose.
predicting which group will preferentially react when a
limited amount of reagent is available.
In the following section, four types of hydroxyl group the sugar acetate at 0 C. Most esters are also saponifi-
◦
derivatives will be discussed: esters, ethers, anhydro sug- able with NaOH in acetone at low temperature. Acetyl,
ars and disaccharides, and cyclic acetals. benzoyl, and p-nitrobenzoyl groups attached to the oxy-
gen atom of the anomeric centers of cyclic sugars can be
a. Formation of esters. Esters are generally used to displaced by nucleophiles, especially in the presence of
block hydroxyl groups, that is, to deactivate their oxy- Lewis acid catalysts. This reaction is useful in the prepara-
gen atoms and, by so doing, prevent them from attacking tion of glycosides and nucleosides. Ester groups attached
nucleophile acceptors. The esters most commonly used to the other carbon atoms of a saccharide, that is, linked
for this purpose are the acetates and benzoates. Occasion- to nonanomeric carbon atoms, are considerably more in-
ally, substituents are introduced in the para position of the ert toward nucleophiles and will not undergo substitution
phenyl ring of the latter esters in order to increase their reactions under mild reaction conditions. To achieve a nu-
crystallizing properties. The O-p-nitrobenzoyl and the O- cleophilic substitution of esters attached to nonanomeric
p-toluoyl derivatives have been found to be useful in this centers, the latter esters must themselves be good leav-
respect. ing groups, for example, tosylates, mesylates, and triflates
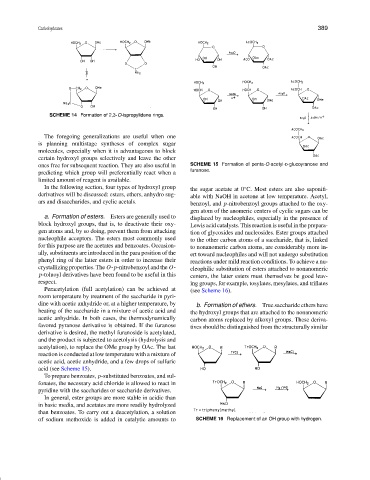
Peracetylation (full acetylation) can be achieved at (see Scheme 16).
room temperature by treatment of the saccharide in pyri-
dine with acetic anhydride or, at a higher temperature, by b. Formation of ethers. True saccharide ethers have
heating of the saccharide in a mixture of acetic acid and the hydroxyl groups that are attached to the nonanomeric
acetic anhydride. In both cases, the thermodynamically carbon atoms replaced by alkoxyl groups. These deriva-
favored pyranose derivative is obtained. If the furanose tives should be distinguished from the structurally similar
derivative is desired, the methyl furanoside is acetylated,
and the product is subjected to acetolysis (hydrolysis and
acetylation), to replace the OMe group by OAc. The last
reaction is conducted at low temperature with a mixture of
acetic acid, acetic anhydride, and a few drops of sulfuric
acid (see Scheme 15).
To prepare benzoates, p-substituted benzoates, and sul-
fonates, the necessary acid chloride is allowed to react in
pyridine with the saccharides or saccharide derivatives.
In general, ester groups are more stable in acidic than
in basic media, and acetates are more readily hydrolyzed
than benzoates. To carry out a deacetylation, a solution
of sodium methoxide is added in catalytic amounts to SCHEME 16 Replacement of an OH group with hydrogen.