Page 78 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 78
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
386 Carbohydrates
depend on the nucleophilicity of the attacking group and products of the Fischer reaction revealed the following
the nature of the leaving group, that is, how good a leaving points. (1) At equilibrium, there is usually a preponder-
group it is. ance of pyranose forms, since the six-membered rings are
The nucleophilic substitution reactions that will be con- the thermodynamically favored forms of glycosides. (2)
sidered in this section are (1) the displacement of the hemi- As a rule, aldoses having adjacent bulky groups (OH or
acetal hydroxyl group ( OH) or one of its esters, for ex- OMe groups) in the cis orientation will afford a smaller
ample, an anomeric acetoxyl group ( O CO R), by an proportion furanosides on equilibration. This is because
OR group from an alcohol to afford glycosides, or by the these substituents are closer together (and repel one an-
X group of hydrogen halides to afford glycosyl halides; other more) in furanosides than in pyranosides. For the
and (2) the displacement of the OR groups of glycosides same reason, a trans relationship of adjacent bulky groups
by OH groups (hydrolysis), by OR groups (anomerization is more favorable in furanosides than in pyranosides. This
and transglycosidation), or by a halogen. is why furanosides having C-1 and C-2 trans (e.g., the
α anomers of D-arabinose and D-lyxose and the β anomers
a. Displacement of OH groups by OR groups of D-ribose and D-xylose) are more stable and exceed the
(glycosidation). The anomeric hydroxyl groups of concentration of their anomers. It was also found that the
cyclic sugars and their esters can be exchanged in acid all-trans-methyl β-D-arabinofuranoside is present in high
media by the OR group of alcohols and phenols. The dis- concentration in the equilibrium mixture. In the case of the
placement of the hemiacetal hydroxyl group by an OR methylated derivatives of D-arabinose (which have bulkier
group is known as the Fischer method of glycoside for- OMegroups),theall-transorientationissofavorableinthe
mation, and the similar exchange of an anomeric ester five-membered ring that, at equilibrium, the concentration
group is referred to as the Helferich method. of the furanosides exceeds that of the pyranosides. (3) Be-
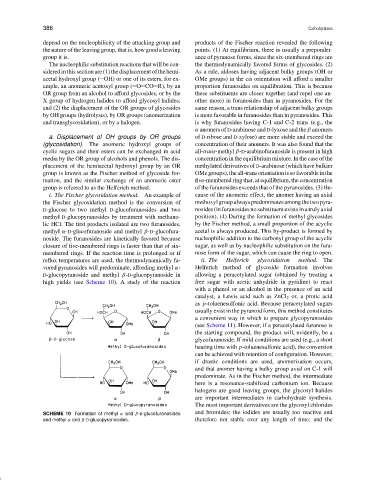
i. The Fischer glycosidation method. An example of cause of the anomeric effect, the anomer having an axial
the Fischer glycosidation method is the conversion of methoxylgroupalwayspredominatesamongthetwopyra-
D-glucose to two methyl D-glucofuranosides and two nosides (in furanosides no substituent exists in a truly axial
methyl D-glucopyranosides by treatment with methano- position). (4) During the formation of methyl glycosides
lic HCl. The first products isolated are two furanosides, by the Fischer method, a small proportion of the acyclic
methyl α-D-glucofuranoside and methyl β-D-glucofura- acetal is always produced. This by-product is formed by
noside. The furanosides are kinetically favored because nucleophilic addition to the carbonyl group of the acyclic
closure of five-membered rings is faster than that of six- sugar, as well as by nucleophilic substitution on the fura-
membered rings. If the reaction time is prolonged or if nose form of the sugar, which can cause the ring to open.
reflux temperatures are used, the thermodynamically fa- ii. The Helferich glycosidation method. The
vored pyranosides will predominate, affording methyl α- Helferich method of glycoside formation involves
D-glucopyranoside and methyl β-D-glucopyranoside in allowing a peracetylated sugar (obtained by treating a
high yields (see Scheme 10). A study of the reaction free sugar with acetic anhydride in pyridine) to react
with a phenol or an alcohol in the presence of an acid
catalyst, a Lewis acid such as ZnCl 2 or, a protic acid
as p-toluenesulfonic acid. Because peracetylated sugars
usually exist in the pyranoid form, this method constitutes
a convenient way in which to prepare glycopyranosides
(see Scheme 11). However, if a peracetylated furanose is
the starting compound, the product will, evidently, be a
glycofuranoside. If mild conditions are used (e.g., a short
heating time with p-toluenesulfonic acid), the conversion
can be achieved with retention of configuration. However,
if drastic conditions are used, anomerization occurs,
and that anomer having a bulky group axial on C-1 will
predominate. As in the Fischer method, the intermediate
here is a resonance-stabilized carbonium ion. Because
halogens are good leaving groups, the glycosyl halides
are important intermediates in carbohydrate synthesis.
The most important derivatives are the glycosyl chlorides
SCHEME 10 Formation of methyl α and β-D-glucofuranosides and bromides; the iodides are usually too reactive and
and methyl α and β-D-glucopyranosides. therefore not stable over any length of time; and the