Page 74 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 74
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
382 Carbohydrates
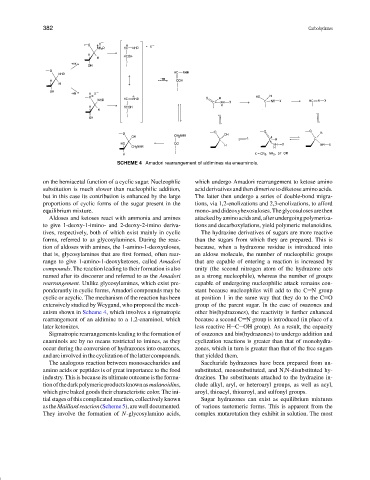
SCHEME 4 Amadori rearrangement of aldimines via eneaminols.
on the hemiacetal function of a cyclic sugar. Nucleophlic which undergo Amadori rearrangement to ketose amino
substitution is much slower than nucleophilic addition, acid derivatives and then dimerize to diketose amino acids.
but in this case its contribution is enhanced by the large The latter then undergo a series of double-bond migra-
proportions of cyclic forms of the sugar present in the tions, via 1,2-enolizations and 2,3-enolizations, to afford
equilibrium mixture. mono-anddideoxyhexosuloses.Theglycosulosesarethen
Aldoses and ketoses react with ammonia and amines attackedbyaminoacidsand,afterundergoingpolymeriza-
to give 1-deoxy-1-imino- and 2-deoxy-2-imino deriva- tions and decarboxylations, yield polymeric melanoidins.
tives, respectively, both of which exist mainly in cyclic The hydrazine derivatives of sugars are more reactive
forms, referred to as glycosylamines. During the reac- than the sugars from which they are prepared. This is
tion of aldoses with amines, the 1-amino-1-deoxyuloses, because, when a hydrazone residue is introduced into
that is, glycosylamines that are first formed, often rear- an aldose molecule, the number of nucleophilic groups
range to give 1-amino-1-deoxyketoses, called Amadori that are capable of entering a reaction is increased by
compounds. The reaction leading to their formation is also unity (the second nitrogen atom of the hydrazone acts
named after its discoerer and referred to as the Amadori as a strong nucleophile), whereas the number of groups
rearrangement. Unlike glycosylamines, which exist pre- capable of undergoing nucleophilic attack remains con-
ponderantly in cyclic forms, Amadori compounds may be stant because nucleophiles will add to the C N group
cyclic or acyclic. The mechanism of the reaction has been at position 1 in the same way that they do to the C O
extensively studied by Weygand, who proposed the mech- group of the parent sugar. In the case of osazones and
anism shown in Scheme 4, which involves a sigmatropic other bis(hydrazones), the reactivity is further enhanced
rearrangement of an aldimine to a 1,2-enaminol, which because a second C N group is introduced (in place of a
later ketonizes. less reactive H C OH group). As a result, the capacity
Sigmatropic rearrangements leading to the formation of of osazones and bis(hydrazones) to undergo addition and
enaminols are by no means restricted to imines, as they cyclization reactions is greater than that of monohydra-
occur during the conversion of hydrazones into osazones, zones, which in turn is greater than that of the free sugars
and are involved in the cyclization of the latter compounds. that yielded them.
The analogous reaction between monosaccharides and Saccharide hydrazones have been prepared from un-
amino acids or peptides is of great importance to the food substituted, monosubstituted, and N,N-disubstituted hy-
industry. This is because its ultimate outcome is the forma- drazines. The substituents attached to the hydrazine in-
tionofthedarkpolymericproductsknownas malanoidins, clude alkyl, aryl, or heteroaryl groups, as well as acyl,
which give baked goods their characteristic color. The ini- aroyl, thioacyl, thioaroyl, and sulfonyl groups.
tial stages of this complicated reaction, collectively known Sugar hydrazones can exist as equilibrium mixtures
as the Maillard reaction (Scheme 5), are well documented. of various tautomeric forms. This is apparent from the
They involve the formation of N-glycosylamino acids, complex mutarotation they exhibit in solution. The most