Page 70 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 70
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
378 Carbohydrates
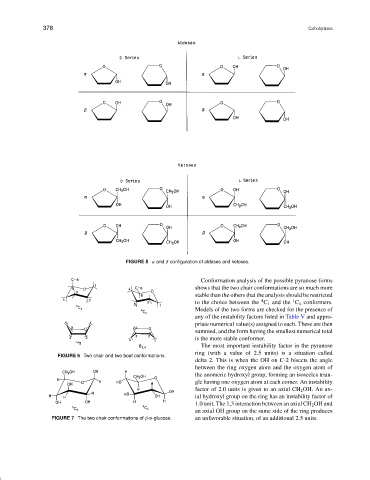
FIGURE 5 α and β configuration of aldoses and ketoses.
Conformation analysis of the possible pyranose forms
shows that the two chair conformations are so much more
stable than the others that the analysis should be restricted
1
4
to the choice between the C 1 and the C 4 conformers.
Models of the two forms are checked for the presence of
any of the instability factors listed in Table V and appro-
priate numerical value(s) assigned to each. These are then
summed, and the form having the smallest numerical total
is the more stable conformer.
The most important instability factor in the pyranose
ring (with a value of 2.5 units) is a situation called
FIGURE 6 Two chair and two boat conformations.
delta 2. This is when the OH on C-2 bisects the angle
between the ring oxygen atom and the oxygen atom of
the anomeric hydroxyl group, forming an isosceles trian-
gle having one oxygen atom at each corner. An instability
factor of 2.0 units is given to an axial CH 2 OH. An ax-
ial hydroxyl group on the ring has an instability factor of
1.0 unit. The 1,3 interaction between an axial CH 2 OH and
an axial OH group on the same side of the ring produces
FIGURE 7 The two chair conformations of β-D-glucose. an unfavorable situation, of an additional 2.5 units.