Page 69 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 69
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
Carbohydrates 377
◦
◦
having 108 angles or a hexagon with 120 angles is rep-
resented as seen by an observer situated at an angle of
◦
∼60 above the plane of ring, and as a precaution against
optical illusions, regarding the side closer to the viewer,
the bonds nearest to the observer are thickened, to give a
sense of perspective (see Fig. 5).
Although rings may be turned around their centers, it is
customary to orient them in such a manner that C-1 is to
the right, and the ring oxygen is farthest from the viewer.
3. Conformation
Pyranose rings can exist in a number of inter-convertible
conformers, of which the chair forms are the most stable.
The number of recognized forms of the pyranose ring are
two chair (C), six boat (B), four half-chair (H), six skew
(S), and six sofa forms. (See Fig. 6 for the two chair and
two of the boat forms.) To designate each of these forms,
the number of the ring atom(s) lying above the plane of the
pyranose ring is superscripted before the letter designating
the form (C, B, H, S, etc.), and the number of the ring
atom(s)lyingbelowtheplaneissubscriptedaftertheletter,
4
e.g., C 1 (see Fig. 7).
It is possible to determine the conformation of a saccha-
ride or glycoside either experimentally or by determining
on purely theoretical grounds which conformer of a given
ring (pyranose or furanose) will be the most stable.
If the conformation in the solid state is desired, X-ray
crystallography or neutron diffraction is prescribed. It will
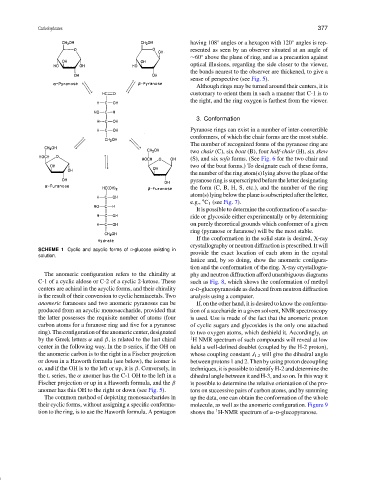
SCHEME 1 Cyclic and acyclic forms of D-glucose existing in
provide the exact location of each atom in the crystal
solution.
lattice and, by so doing, show the anomeric configura-
tion and the conformation of the ring. X-ray crystallogra-
The anomeric configuration refers to the chirality at phy and neutron diffraction afford unambiguous diagrams
C-1 of a cyclic aldose or C-2 of a cyclic 2-ketose. These such as Fig. 8, which shows the conformation of methyl
centers are achiral in the acyclic forms, and their chirality α-D-glucopyranoside as deduced from neutron diffraction
is the result of their conversion to cyclic hemiacetals. Two analysis using a computer.
anomeric furanoses and two anomeric pyranoses can be If, on the other hand, it is desired to know the conforma-
produced from an acyclic monosaccharide, provided that tion of a saccharide in a given solvent, NMR spectroscopy
the latter possesses the requisite number of atoms (four is used. Use is made of the fact that the anomeric proton
carbon atoms for a furanose ring and five for a pyranose of cyclic sugars and glycosides is the only one attached
ring).Theconfigurationoftheanomericcenter,designated to two oxygen atoms, which deshield it. Accordingly, an
by the Greek letters α and β, is related to the last chiral 1 H NMR spectrum of such compounds will reveal at low
center in the following way. In the D series, if the OH on field a well-defined doublet (coupled by the H-2 proton),
the anomeric carbon is to the right in a Fischer projection whose coupling constant J 1.2 will give the dihedral angle
or down in a Haworth formula (see below), the isomer is between protons 1 and 2. Then by using proton decoupling
α, and if the OH is to the left or up, it is β. Conversely, in techniques, it is possible to identify H-2 and determine the
the L series, the α anomer has the C-1 OH to the left in a dihedral angle between it and H-3, and so on. In this way it
Fischer projection or up in a Haworth formula, and the β is possible to determine the relative orientation of the pro-
anomer has this OH to the right or down (see Fig. 5). tons on successive pairs of carbon atoms, and by summing
The common method of depicting monosaccharides in up the data, one can obtain the conformation of the whole
their cyclic forms, without assigning a specific conforma- molecule, as well as the anomeric configuration. Figure 9
1
tion to the ring, is to use the Haworth formula. A pentagon shows the H-NMR spectrum of α-D-glucopyranose.