Page 64 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 64
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
372 Carbohydrates
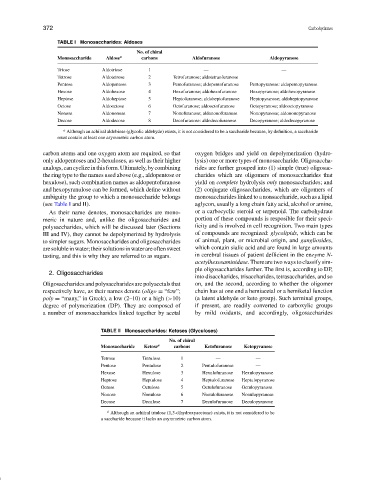
TABLE I Monosaccharides: Aldoses
No. of chiral
Monosaccharide Aldose a carbons Aldofuranose Aldopyranose
Triose Aldotriose 1 — —
Tetrose Aldotetrose 2 Tetrofuranose; aldotetraofuranose —
Pentose Aldopentose 3 Pentofuranose; aldopentofuranose Pentopyranose: aldopentopyranose
Hexose Aldohexose 4 Hexofuranose; aldohexofuranose Hexopyranose; aldohexopyranose
Heptose Aldoheptose 5 Heptofuranose; aldoheptofuranose Heptopyranose; aldoheptopyranose
Octose Aldooctose 6 Octofuranose; aldooctofuranose Octopyranose; aldooctopyranose
Nonose Aldononose 7 Nonofuranose; aldononofuranose Nonopyranose; aldononopyranose
Decose Aldodecose 8 Decofuranose: aldodecofuranose Decopyranose; aldodecopyranose
a Although an achiiral aldobiose (glycolic aldehyde) exists, it is not considered to be a saccharide because, by definition, a saccharide
must contain at least one asymmetric carbon atom.
carbon atoms and one oxygen atom are required, so that oxygen bridges and yield on depolymerization (hydro-
only aldopentoses and 2-hexuloses, as well as their higher lysis) one or more types of monosaccharide. Oligosaccha-
analogs,cancyclizeinthisform.Ultimately,bycombining rides are further grouped into (1) simple (true) oligosac-
the ring type to the names used above (e.g., aldopentose or charides which are oligomers of monosaccharides that
hexulose), such combination names as aldopentofuranose yield on complete hydrolysis only monosaccharides; and
and hexopyranulose can be formed, which define without (2) conjugate oligosaccharides, which are oligomers of
ambiguity the group to which a monosaccharide belongs monosaccharides linked to a nonsaccharide, such as a lipid
(see Table I and II). aglycon, usually a long chain fatty acid, alcohol or amine,
As their name denotes, monosaccharides are mono- or a carbocyclic steroid or terpenoid. The carbohydrate
meric in nature and, unlike the oligosaccharides and portion of these compounds is resposible for their speci-
polysaccharides, which will be discussed later (Sections ficity and is involved in cell recognition. Two main types
III and IV), they cannot be depolymerized by hydrolysis of compounds are recognized: glycolipids, which can be
to simpler sugars. Monosaccharides and oligosaccharides of animal, plant, or microbial origin, and gangliosides,
aresolubleinwater;theirsolutionsinwaterareoftensweet which contain sialic acid and are found in large amounts
tasting, and this is why they are referred to as sugars. in cerebral tissues of patient defficient in the enzyme N-
acetylhexosaminidase. There are two ways to classify sim-
ple oligosaccharides further. The first is, according to DP,
2. Oligosaccharides
into disaccharides, trisaccharides, tetrasaccharides, and so
Oligosaccharides and polysaccharides are polyacetals that on, and the second, according to whether the oligomer
respectively have, as their names denote (oligo = “few”; chain has at one end a hemiacetal or a hemiketal function
poly = “many,” in Greek), a low (2–10) or a high (>10) (a latent aldehyde or keto group). Such terminal groups,
degree of polymerization (DP). They are composed of if present, are readily converted to carboxylic groups
a number of monosaccharides linked together by acetal by mild oxidants, and accordingly, oligosaccharides
TABLE II Monosaccharides: Ketoses (Glyculoses)
No. of chiral
Monosaccharide Ketose a carbons Ketofuranose Ketopyranose
Tetrose Tetrulose 1 — —
Pentose Pentulose 2 Pentulofuranose —
Hexose Hexulose 3 Hexulofuranose Hexulopyranose
Heptose Heptulose 4 Heptulofuranose Heptulopyranose
Octose Octulose 5 Octulofuranose Octulopyranose
Nonose Nonulose 6 Nonulofuranose Nonulopyranose
Decose Deculose 7 Deculofuranose Deculopyranose
a Although an achiiral triulose (1,3-dihydroxyacetone) exists, it is not considered to be
a saccharide because it lacks an asymmetric carbon atom.