Page 68 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 68
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
376 Carbohydrates
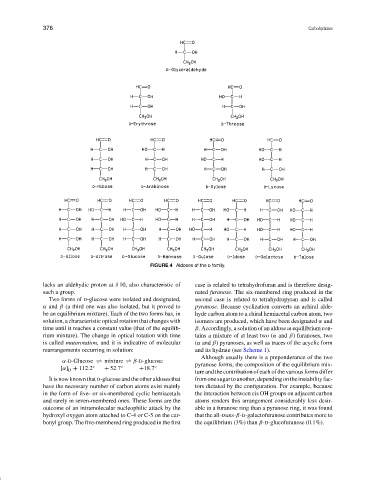
FIGURE 4 Aldoses of the D family.
lacks an aldehydic proton at δ 10, also characteristic of case is related to tetrahydrofuran and is therefore desig-
such a group. nated furanose. The six-membered ring produced in the
Two forms of D-glucose were isolated and designated, second case is related to tetrahydropyran and is called
α and β (a third one was also isolated, but it proved to pyranose. Because cyclization converts an achiral alde-
be an equilibrium mixture). Each of the two forms has, in hyde carbon atom to a chiral hemiacetal carbon atom, two
solution, a characteristic optical rotation that changes with isomers are produced, which have been designated α and
time until it reaches a constant value (that of the equilib- β. Accordingly, a solution of an aldose at equilibrium con-
rium mixture). The change in optical rotation with time tains a mixture of at least two (α and β) furanoses, two
is called mutarotation, and it is indicative of molecular (α and β) pyranoses, as well as traces of the acyclic form
rearrangements occurring in solution: and its hydrate (see Scheme 1).
Although usually there is a preponderance of the two
α-D-Glucose mixture β-D-glucose
pyranose forms, the composition of the equilibrium mix-
[α] D + 112.2 ◦ + 52.7 ◦ +18.7 ◦
ture and the contribution of each of the various forms differ
Itisnowknownthat D-glucoseand the other aldoses that fromonesugartoanother,dependingontheinstabilityfac-
have the necessary number of carbon atoms exist mainly tors dictated by the configuration. For example, because
in the form of five- or six-membered cyclic hemiacetals the interaction between cis OH groups on adjacent carbon
and rarely in seven-membered ones. These forms are the atoms renders this arrangement considerably less desir-
outcome of an intramolecular nucleophilic attack by the able in a furanose ring than a pyranose ring, it was found
hydroxyl oxygen atom attached to C-4 or C-5 on the car- that the all-trans-β-D-galactofuranose contributes more to
bonyl group. The five-membered ring produced in the first the equilibrium (3%) than β-D-glucofuranose (0.1%).