Page 137 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 137
P1: GLM/GJK P2: GRB Final Pages
Encyclopedia of Physical Science and Technology En005H-218 June 15, 2001 20:33
Electrophoresis 365
aa
θ +
∴ T + = =
aa + bb
u + + u −
and
bb
T − = . (5)
aa + bb
If this experiment were performed, the sharpness of the
boundaries would be affected by the concentrations of the
three salts [see Eq. (4)]. A sharp boundary would form
−
+
+
only if Li did not overtake K and Ac did not overtake
−
Cl ; at the limit this means that the velocity of Li would
+
be the same as that of K and the velocity of Ac would
+
−
equalthatofCl .Forasetoftheseboundaries,Kohlrausch
−
defined a regulating function for defining sharpening con-
ditions, namely, when T + /C + is equal on both sides of the
boundary for each ion of the same sign, a sharp boundary
forms. This function can be derived from Eq. (4) if one
remembers that the sum in the denominator represents
the total conductance; hence, the mobilities must be equal
(2)
if T (1) /C (1) = T (2) /C ; this condition is enlisted to give
+ + + +
stacking of proteins in gel electrophoresis (Section III.D).
Boundary experiments were employed during the early
part of the 20th century for studying aqueous salt solu-
tions, and the rules that emerged are associated with such
people as Hittorf and McBain. The results showed that
ions were hydrated not equally, but by an amount that
appeared to be linearly related to the size of the ions.
Most of these studies were made using inorganic ions,
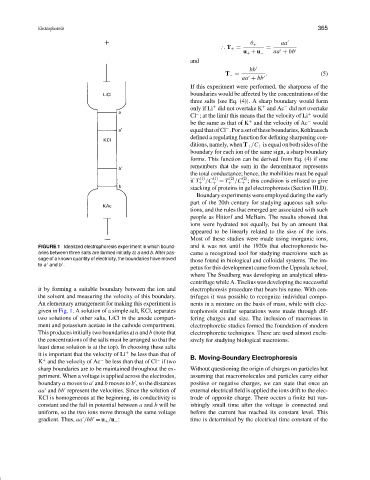
FIGURE 1 Idealized electrophoresis experiment in which bound- and it was not until the 1920s that electrophoresis be-
aries between three salts are formed initially at a and b. After pas- came a recognized tool for studying macroions such as
sage of a known quantity of electricity, the boundaries have moved
those found in biological and colloidal systems. The im-
to a and b .
petus for this development came from the Uppsala school,
where The Svedberg was developing an analytical ultra-
centrifuge while A. Tiselius was developing the successful
it by forming a suitable boundary between the ion and electrophoresis procedure that bears his name. With cen-
the solvent and measuring the velocity of this boundary. trifuges it was possible to recognize individual compo-
An elementary arrangement for making this experiment is nents in a mixture on the basis of mass, while with elec-
given in Fig. 1. A solution of a simple salt, KCl, separates trophoresis similar separations were made through dif-
two solutions of other salts, LiCl in the anode compart- fering charges and size. The inclusion of macroions in
ment and potassium acetate in the cathode compartment. electrophoretic studies formed the foundation of modern
This produces initially two boundaries at a and b (note that electrophoretic techniques. These are used almost exclu-
the concentrations of the salts must be arranged so that the sively for studying biological macroions.
least dense solution is at the top). In choosing these salts
+
it is important that the velocity of Li be less than that of B. Moving-Boundary Electrophoresis
K and the velocity of Ac be less than that of Cl if two
−
+
−
sharp boundaries are to be maintained throughout the ex- Without questioning the origin of charges on particles but
periment. When a voltage is applied across the electrodes, assuming that macromolecules and particles carry either
boundary a moves to a and b moves to b , so the distances positive or negative charges, we can state that once an
aa and bb represent the velocities. Since the solution of external electrical field is applied the ions drift to the elec-
KCl is homogeneous at the beginning, its conductivity is trode of opposite charge. There occurs a finite but van-
constant and the fall in potential between a and b will be ishingly small time after the voltage is connected and
uniform, so the two ions move through the same voltage before the current has reached its constant level. This
gradient. Thus, aa /bb = u + /u − : time is determined by the electrical time constant of the