Page 178 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 178
P1: GQT Final
Encyclopedia of Physical Science and Technology EN006F-275 June 29, 2001 21:12
Gas Chromatography 469
analysis and unattended operation of the instrument. to use appropriate standard compounds. Some automated
Moreover, reproducibility of the sample injection is im- analyzers based on GC principles are also used in the pro-
proved considerably. cess control and continuous analysis of industrial streams.
A precise column temperature control is now required Specialized GC techniques find their place in scientific
for all commercial gas chromatographs. In practice, the research. The extremely high sensitivities of some GC de-
GC ovens are designed to have low thermal mass. Resis- tectors are unparalleled.
tance spirals situated inside the oven are proportionally The GC method is employed for a variety of mixtures,
heated, while the air circulation throughout the oven is ranging from permanent gases up to molecules that are al-
provided by a fan. For adequate analytical work, the col- most as large as 1000 Da of molecular weight. The variety
umn temperature should be reproducible within at least ofchromatographiccolumnsanddetectorsavailabletoGC
◦
±0.1 C. continues to expand its applicability to various analytical
Retention times in GC are affected by temperature. In problems. Several representative examples will now be de-
accordance with Eqs. (1) and (2), the retention time de- scribed to demonstrate the method’s versatility, resolving
creases with increasing temperature because the partition power, selectivity, and sensitivity. These examples have
coefficient is decreased. Various solutes, depending on been chosen from the areas of industrial analysis, occu-
their structures and the chemical nature of a particular pational hygiene, and biochemical research. Other major
phase system, have different dependencies on tempera- areas, not covered here, are geochemistry, food and aroma
ture. Consequently, temperature optimization is necessary analysis, various agricultural and environmental analyses,
for the maximum resolution of the analyzed components. atmospheric measurements, and forensic investigations.
For the mixtures of components with very different val- The analysis of light gases (permanent gases, gaseous
ues of partition coefficient, column temperature program- oxides, and C 1 –C 5 hydrocarbons) has been traditionally
ming is often employed. Commercial gas chromatographs performed in gas–solid chromatographic systems. Vari-
are equipped with convective heating systems that facili- ous porous adsorbents possess the capability to adsorb and
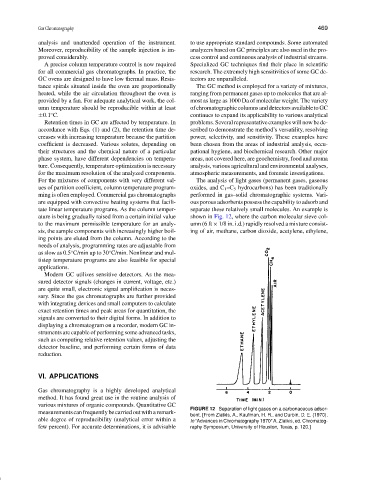
tate linear temperature programs. As the column temper- separate these relatively small molecules. An example is
ature is being gradually raised from a certain initial value shown in Fig. 12, where the carbon molecular sieve col-
to the maximum permissible temperature for an analy- umn (6 ft × 1/8 in. i.d.) rapidly resolved a mixture consist-
sis, the sample components with increasingly higher boil- ing of air, methane, carbon dioxide, acetylene, ethylene,
ing points are eluted from the column. According to the
needs of analysis, programming rates are adjustable from
◦
◦
as slow as 0.5 C/min up to 30 C/min. Nonlinear and mul-
tistep temperature programs are also feasible for special
applications.
Modern GC utilizes sensitive detectors. As the mea-
sured detector signals (changes in current, voltage, etc.)
are quite small, electronic signal amplification is neces-
sary. Since the gas chromatographs are further provided
with integrating devices and small computers to calculate
exact retention times and peak areas for quantitation, the
signals are converted to their digital forms. In addition to
displaying a chromatogram on a recorder, modern GC in-
struments are capable of performing some advanced tasks,
such as computing relative retention values, adjusting the
detector baseline, and performing certain forms of data
reduction.
VI. APPLICATIONS
Gas chromatography is a highly developed analytical
method. It has found great use in the routine analysis of
various mixtures of organic compounds. Quantitative GC
FIGURE 12 Separation of light gases on a carbonaceous adsor-
measurementscanfrequentlybecarriedoutwitharemark-
bent. [From Zlatkis, A., Kaufman, H. R., and Durbin, D. E. (1970).
able degree of reproducibility (analytical error within a In “Advances in Chromatography 1970” A. Zlatkis, ed. Chromatog-
few percent). For accurate determinations, it is advisable raphy Symposium, University of Houston, Texas, p. 120.]