Page 292 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 292
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009N-447 July 19, 2001 23:3
Microwave Molecular Spectroscopy 809
TABLE IV Spectroscopic Constants and Structures for
Some Symmetric-Top Molecules
Molecule B 0 (MHz) D j (kHz) D JK (kHz)
PH 3 133,480.15 3950 −5180
AsH 3 112,470.59 2925 −3718
CH 3 F 25,536.15 59.9 420.3
CH 3 CN 9,198.90 3.8 176.9
SiH 3 CN 4,973.01 1.5 63
OPF 3 4,811.76 1.0 1.3
CH 3 CN···HF 1,853.37 0.8 67
C 6 C 6 ···HCl 1,237.68 1.2 13.3
Bond Bond
Molecule Bond length ( ˚ A) Angle angle (deg)
PH 3 P H 1.420 HPH 93.3
AsH 3 As H 1.520 HAsH 92.0
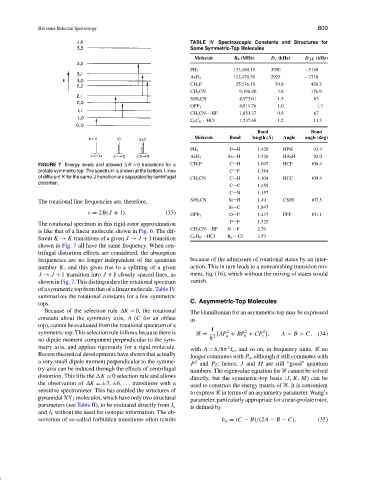
FIGURE 7 Energy levels and allowed K = 0 transitions for a CH 3 F C H 1.097 HCF 108.4
prolate symmetric top. The spectrum is shown at the bottom. Lines C F 1.384
of different K for the same J transition are separated by centrifugal CH 3 CN C H 1.104 HCC 109.4
distortion.
C C 1.458
C N 1.157
The rotational line frequencies are, therefore, SiH 3 CN Si H 1.49 CSiH 107.5
Si C 1.847
ν = 2B(J + 1). (33) O P 1.437 FPF 101.1
OPF 3
The rotational spectrum in this rigid-rotor approximation P F 1.522
is like that of a linear molecule shown in Fig. 6. The dif- CH 3 CN···HF N···F 2.76
ferent K → K transitions of a given J → J + 1 transition C 6 H 6 ···HCl B z ···Cl 3.59
shown in Fig. 7 all have the same frequency. When cen-
trifugal distortion effects are considered, the absorption
frequencies are no longer independent of the quantum because of the admixture of rotational states by an inter-
number K, and this gives rise to a splitting of a given action. This in turn leads to a nonvanishing transition mo-
J → J + 1 transition into J + 1 closely spaced lines, as ment, Eq. (16), which without the mixing of states would
shown in Fig. 7. This distinguishes the rotational spectrum vanish.
of a symmetric top from that of a linear molecule. Table IV
summarizes the rotational constants for a few symmetric
C. Asymmetric-Top Molecules
tops.
Because of the selection rule K = 0, the rotational The Hamiltonian for an asymmetric top may be expressed
constant about the symmetry axis, A (C for an oblate as
top), cannot be evaluated from the rotational spectrum of a
2
2
symmetric top. This selection rule follows because there is = 1 AP + BP + CP , A > B > C, (34)
2
c
b
a
2
no dipole moment component perpendicular to the sym- h
metry axis, and applies rigorously for a rigid molecule. with A = h/8π I a , and so on, in frequency units. no
2
Recent theoretical developments have shown that actually longer commutes with P a , although it still commutes with
a very small dipole moment perpendicular to the symme- P and P Z ; hence, J and M are still “good” quantum
2
try axis can be induced through the effects of centrifugal
numbers. The eigenvalue equation for cannot be solved
distortion. This lifts the K = 0 selection rule and allows
directly, but the symmetric-top basis |J, K, M) can be
the observation of K =±3, ±6,... transitions with a
used to construct the energy matrix of . It is convenient
sensitive spectrometer. This has enabled the structures of
to express in terms of an asymmetry parameter. Wang’s
pyramidal XY 3 molecules, which have only two structural
parameter,particularlyappropriateforanear-prolaterotor,
is defined by
parameters (see Table II), to be evaluated directly from I a
and I b without the need for isotopic information. The ob-
servation of so-called forbidden transitions often results b p = (C − B)/(2A − B − C), (35)