Page 289 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 289
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009N-447 July 19, 2001 23:3
806 Microwave Molecular Spectroscopy
axis system relative to the initial arbitary axis system. tailed molecular structures. Because of the sensitivity of
Equivalently, the secular determinant microwave spectroscopy, various isotopic forms can often
be studied in natural abundance.
xx − λ I xy I xz
I
IV. RIGID-ROTOR ENERGY LEVELS
I xy I yy − λ I yz = 0 (14)
AND SPECTRA
I xz I yz I zz − λ
may be expanded and solved for its roots, which are the To a good approximation, the energy of a molecule may
principal moments. In general, the above is of third degree be expressed as the sum of the electronic, vibrational, and
in the unknown λ. If, however, one principal axis is known, rotational energies. In pure rotational spectra, transitions
then by taking one of the initial coordinate axes along this take place between rotational sublevels with no change in
direction, one can make the products of inertia associated the electronic or vibrational state. The gross features of the
withthisaxisvanish,andthesecularequationissimplified. pure rotational spectrum of a molecule may be ascertained
The masses employed in calculating the moments and bytreatingthemoleculeasarigid,nonvibratingrotor.Sub-
products of inertia must correspond to a single isotope for sequently, the effects of centrifugal distortion and vibra-
each atom in the molecule. Since the principal moments tion may, in many cases, be included by application of per-
of inertia are different for different isotopic forms of a turbation theory. This is considered further in Sections V
molecule, quite different rotational spectra are obtained. and VI. The molecular geometry, via the moments of in-
In fact, if the molecular mass distribution in a molecule ertia, determines the pattern of the rigid-rotor spectrum.
is changed, the rotational spectrum is affected. The spec- This pattern is relatively simple for linear and symmetric-
trum of 2-chloropyridine can, hence, be expected to be top molecules; however, for asymmetric rotors, there is
quite different from that of 3-chloropyridine. The impli- little regularity to the spectrum except in certain cases.
cations for qualitative analysis of these chemical isomers The frequency of electromagnetic radiation absorbed
are obvious. Rotational isomerism also changes the mass depends on the energy difference between the two states
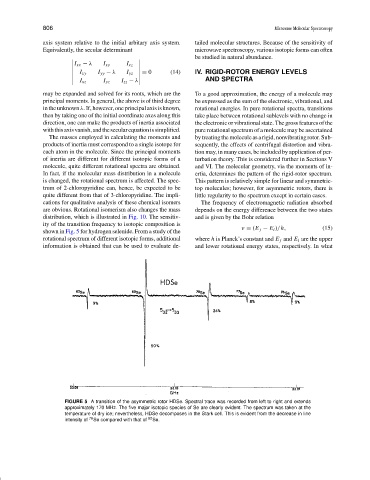
distribution, which is illustrated in Fig. 10. The sensitiv- and is given by the Bohr relation
ity of the transition frequency to isotopic composition is
ν = (E j − E i )/h, (15)
shown in Fig. 5 for hydrogen selenide. From a study of the
rotational spectrum of different isotopic forms, additional where h is Planck’s constant and E j and E i are the upper
information is obtained that can be used to evaluate de- and lower rotational energy states, respectively. In what
FIGURE 5 A transition of the asymmetric rotor HDSe. Spectral trace was recorded from left to right and extends
approximately 170 MHz. The five major isotopic species of Se are clearly evident. The spectrum was taken at the
temperature of dry ice; nevertheless, HDSe decomposes in the Stark cell. This is evident from the decrease in line
intensity of 76 Se compared with that of 82 Se.