Page 386 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 386
P1: LLL/LOS/GJM P2: GLM Final Pages
Encyclopedia of Physical Science and Technology EN0011A-541 July 25, 2001 17:27
Organic Chemistry, Compound Detection 489
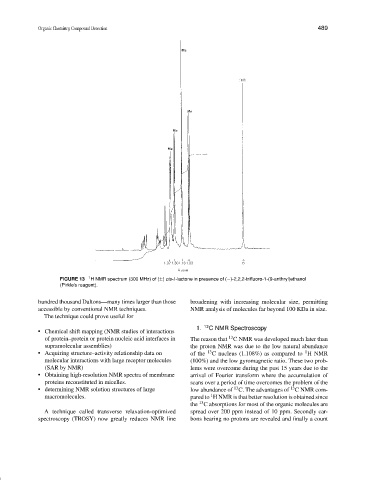
FIGURE 13 1 H NMR spectrum (300 MHz) of (±) cis-δ-lactone in presence of (−)-2,2,2-trifluoro-1-(9-anthryl)ethanol
(Pirkle’s reagent).
hundred thousand Daltons—many times larger than those broadening with increasing molecular size, permitting
accessible by conventional NMR techniques. NMR analysis of molecules far beyond 100 KDa in size.
The technique could prove useful for
1. 13 C NMR Spectroscopy
Chemical shift mapping (NMR studies of interactions
13
of protein–protein or protein nucleic acid interfaces in The reason that C NMR was developed much later than
supramolecular assemblies) the proton NMR was due to the low natural abundance
Acquiring structure–activity relationship data on of the C nucleus (1.108%) as compared to H NMR
13 1
molecular interactions with large receptor molecules (100%) and the low gyromagnetic ratio. These two prob-
(SAR by NMR) lems were overcome during the past 15 years due to the
Obtaining high-resolution NMR spectra of membrane arrival of Fourier transform where the accumulation of
proteins reconstituted in micelles. scans over a period of time overcomes the problem of the
determining NMR solution structures of large low abundance of C. The advantages of C NMR com-
13 13
1
macromolecules. pared to H NMR is that better resolution is obtained since
13
the C absorptions for most of the organic molecules are
A technique called transverse relaxation-optimized spread over 200 ppm instead of 10 ppm. Secondly car-
spectroscopy (TROSY) now greatly reduces NMR line bons bearing no protons are revealed and finally a count