Page 387 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 387
P1: LLL/LOS/GJM P2: GLM Final Pages
Encyclopedia of Physical Science and Technology EN0011A-541 July 25, 2001 17:27
490 Organic Chemistry, Compound Detection
1
of the number of protons attached to each carbon results it is protons from H NMR. Finally protons attached to
13
from comparison of the broad-band decoupled 13 C NMR heteroatoms are not visible. For the time being C NMR
13
spectrum with the off resonance C spectrum. Therefore, spectroscopy is a method that compliments other spec-
the number of methyl (quartet), methylene (triplet), me- troscopic techniques necessary to elucidate structures of
thinyl (doublet), and quarternary (singlet) carbons in a organic molecules. One commercial application of 13 C
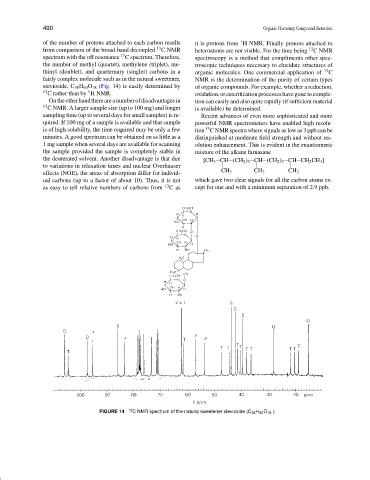
fairly complex molecule such as in the natural sweetener, NMR is the determination of the purity of certain types
stevioside, C 38 H 60 O 18 (Fig. 14) is easily determined by of organic compounds. For example, whether a reduction,
13 1
C rather than by H NMR. oxidation, or esterification processes have gone to comple-
Ontheotherhandthereareanumberofdisadvantagesin tion can easily and also quite rapidly (if sufficient material
13
C NMR. A larger sample size (up to 100 mg) and longer is available) be determined.
sampling time (up to several days for small samples) is re- Recent advances of even more sophisticated and more
quired. If 100 mg of a sample is available and that sample powerful NMR spectrometers have enabled high resolu-
is of high solubility, the time required may be only a few tion C NMR spectra where signals as low as 3 ppb can be
13
minutes. A good spectrum can be obtained on as little as a distinguished at moderate field strength and without res-
1 mg sample when several days are available for scanning olution enhancement. This is evident in the enantiomeric
the sample provided the sample is completely stable in mixture of the alkane farnasane
the deuterated solvent. Another disadvantage is that due [CH 3 CH (CH 2 ) 3 CH (CH 2 ) 3 CH CH 2 CH 3 ]
to variations in relaxation times and nuclear Overhauser | | |
effects (NOE), the areas of absorption differ for individ- CH 3 CH 3 CH 3
ual carbons (up to a factor of about 10). Thus, it is not which gave two clear signals for all the carbon atoms ex-
as easy to tell relative numbers of carbons from 13 Cas cept for one and with a minimum separation of 2.9 ppb.
FIGURE 14 13 C NMR spectrum of the natural sweetener stevioside (C 38 H 60 O 18 ).