Page 111 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 111
P1: GTQ/GUB P2: GSS/GJP QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN010B-472 July 16, 2001 15:41
Natural Antioxidants In Foods 339
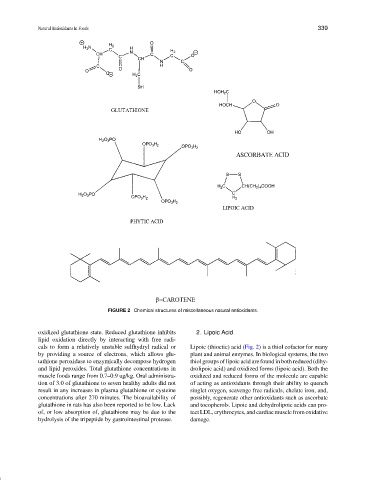
FIGURE 2 Chemical structures of miscellaneous natural antioxidants.
oxidized glutathione state. Reduced glutathione inhibits 2. Lipoic Acid
lipid oxidation directly by interacting with free radi-
cals to form a relatively unstable sulfhydryl radical or Lipoic (thioctic) acid (Fig. 2) is a thiol cofactor for many
by providing a source of electrons, which allows glu- plant and animal enzymes. In biological systems, the two
tathione peroxidase to enzymically decompose hydrogen thiol groups of lipoic acid are found in both reduced (dihy-
and lipid peroxides. Total glutathione concentrations in drolipoic acid) and oxidized forms (lipoic acid). Both the
muscle foods range from 0.7–0.9 ug/kg. Oral administra- oxidized and reduced forms of the molecule are capable
tion of 3.0 of glutathione to seven healthy adults did not of acting as antioxidants through their ability to quench
result in any increases in plasma glutathione or cysteine singlet oxygen, scavenge free radicals, chelate iron, and,
concentrations after 270 minutes. The bioavailability of possibly, regenerate other antioxidants such as ascorbate
glutathione in rats has also been reported to be low. Lack and tocopherols. Lipoic and dehydrolipoic acids can pro-
of, or low absorption of, glutathione may be due to the tect LDL, erythrocytes, and cardiac muscle from oxidative
hydrolysis of the tripeptide by gastrointestinal protease. damage.