Page 108 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 108
P1: GTQ/GUB P2: GSS/GJP QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN010B-472 July 16, 2001 15:41
336 Natural Antioxidants In Foods
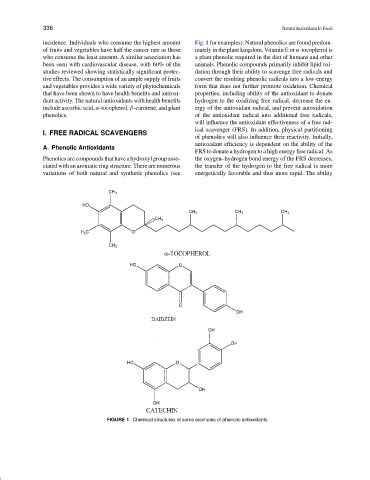
incidence. Individuals who consume the highest amount Fig. 1 for examples). Natural phenolics are found predom-
of fruits and vegetables have half the cancer rate as those inately in the plant kingdom. Vitamin E or α-tocopherol is
who consume the least amount. A similar association has a plant phenolic required in the diet of humans and other
been seen with cardiovascular disease, with 60% of the animals. Phenolic compounds primarily inhibit lipid oxi-
studies reviewed showing statistically significant protec- dation through their ability to scavenge free radicals and
tive effects. The consumption of an ample supply of fruits convert the resulting phenolic radicals into a low-energy
and vegetables provides a wide variety of phytochemicals form that does not further promote oxidation. Chemical
that have been shown to have health benefits and antioxi- properties, including ability of the antioxidant to donate
dant activity. The natural antioxidants with health benefits hydrogen to the oxidizing free radical, decrease the en-
include ascorbic acid, α-tocopherol, β-carotene, and plant ergy of the antioxidant radical, and prevent autoxidation
phenolics. of the antioxidant radical into additional free radicals,
will influence the antioxidant effectiveness of a free rad-
ical scavenger (FRS). In addition, physical partitioning
I. FREE RADICAL SCAVENGERS
of phenolics will also influence their reactivity. Initially,
antioxidant efficiency is dependent on the ability of the
A. Phenolic Antioxidants
FRS to donate a hydrogen to a high energy free radical. As
Phenolics are compounds that have a hydroxyl group asso- the oxygen–hydrogen bond energy of the FRS decreases,
ciated with an aromatic ring structure. There are numerous the transfer of the hydrogen to the free radical is more
variations of both natural and synthetic phenolics (see energetically favorable and thus more rapid. The ability
FIGURE 1 Chemical structures of some examples of phenolic antioxidants.