Page 104 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 104
P1: GST/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN009G-417 July 10, 2001 15:10
Membrane Structure 365
VI. INTERACTION OF MEMBRANE LIPIDS cross-sectional area of the fatty acyl chains. An area ex-
WITH AMPHIPHILIC MOLECULES AND pansion upon membrane penetration of amphiphilic com-
TRANSMEMBRANE PROTEINS pounds was also shown with molecular dynamics simula-
tion for local anesthetics and peptides. The observation of
A. Lipid Order Parameter in the Presence an area increase upon insertion of local anesthetics is con-
of Amphiphilic Molecules sistent with the phenomenon of pressure reversal of local
anesthesia, which may be due to the anisotropic compres-
The outer lipid membrane surface of eukaryotic cells
sion of lipid membranes under hydrostatic pressure and
is generally uncharged. Amphiphilic, water-soluble mol-
the consequent release of anesthetic molecules.
ecules such as local anesthetics, viral or antibiotic pep-
tides, or peptide toxins therefore partition into the bilayer
interface because of their hydrophobicity. All these com- B. Order and Fluidity in the Presence
poundsarefoundtodecreasetheorderoflipidmembranes. of Transmembrane Proteins
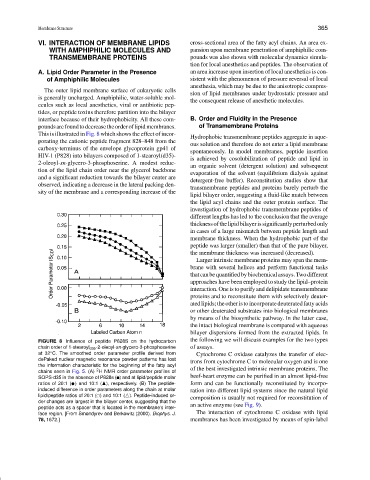
This is illustrated in Fig. 8 which shows the effect of incor-
Hydrophobic transmembrane peptides aggregate in aque-
porating the cationic peptide fragment 828–848 from the
ous solution and therefore do not enter a lipid membrane
carboxy-terminus of the envelope glycoprotein gp41 of
spontaneously. In model membranes, peptide insertion
HIV-1 (P828) into bilayers composed of 1-stearoyl(d35)-
is achieved by cosolubilization of peptide and lipid in
2-oleoyl-sn-glycero-3-phosphoserine. A modest reduc-
an organic solvent (detergent solution) and subsequent
tion of the lipid chain order near the glycerol backbone
evaporation of the solvent (equilibrium dialysis against
and a significant reduction towards the bilayer center are
detergent-free buffer). Reconstitution studies show that
observed, indicating a decrease in the lateral packing den-
transmembrane peptides and proteins barely perturb the
sity of the membrane and a corresponding increase of the
lipid bilayer order, suggesting a fluid-like match between
the lipid acyl chains and the outer protein surface. The
investigation of hydrophobic transmembrane peptides of
different lengths has led to the conclusion that the average
thicknessofthelipidbilayerissignificantlyperturbedonly
in cases of a large mismatch between peptide length and
membrane thickness. When the hydrophobic part of the
peptide was larger (smaller) than that of the pure bilayer,
the membrane thickness was increased (decreased).
Larger intrinsic membrane proteins may span the mem-
brane with several helices and perform functional tasks
thatcanbequantifiedbybiochemicalassays.Twodifferent
approaches have been employed to study the lipid–protein
interaction. One is to purify and delipidate transmembrane
proteins and to reconstitute them with selectively deuter-
atedlipids;theotheristoincorporatedeuteratedfattyacids
or other deuterated substrates into biological membranes
by means of the biosynthetic pathway. In the latter case,
the intact biological membrane is compared with aqueous
bilayer dispersions formed from the extracted lipids. In
FIGURE 8 Influence of peptide P828S on the hydrocarbon the following we will discuss examples for the two types
chain order of 1-stearoyl d35 -2-oleoyl-sn-glycero-3-phosphoserine of assays.
at 32 C. The smoothed order parameter profile derived from Cytochrome C oxidase catalyzes the transfer of elec-
◦
dePaked nuclear magnetic resonance powder patterns has lost
trons from cytochrome C to molecular oxygen and is one
the information characteristic for the beginning of the fatty acyl
2
chains seen in Fig. 5. (A) H NMR order parameter profiles of of the best investigated intrinsic membrane proteins. The
beef-heart enzyme can be purified in an almost lipid-free
SOPS-d35 in the absence of P828s ( ) and at lipid/peptide molar
ratios of 20:1 ( ✉ ) and 10:1 ( ), respectively. (B) The peptide- form and can be functionally reconstituted by incorpo-
induced difference in order parameters along the chain at molar ration into different lipid systems since the natural lipid
lipid/peptide ratios of 20:1 ( ) and 10:1 ( ). Peptide-induced or- composition is usually not required for reconstitution of
❤
der changes are largest in the bilayer center, suggesting that the
peptide acts as a spacer that is located in the membrane’s inter- an active enzyme (see Fig. 9).
face region. [From Smondyrev and Berkowitz (2000). Biophys. J. The interaction of cytochrome C oxidase with lipid
78, 1672.] membranes has been investigated by means of spin-label