Page 99 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 99
P1: GST/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN009G-417 July 10, 2001 15:10
360 Membrane Structure
face moves the N end of the headgroup −P-N dipole
+
+
away from the membrane surface, and a negative charge
+
moves the N end towards the hydrocarbon phase. The
out-of-plane movement of the phospholipid headgroup
dipole creates a local electric field across the membrane,
5
which can easily reach a field strength of 10 V/cm. Such
high electric fields can, in principle, entail conforma-
tional changes of membrane-bound proteins, and the lipid
dipole field could thus play a regulatory role in membrane
function.
If the membrane contains negatively charged lipids to
begin with, the concentration of cationic compounds at
the membrane surface is drastically enhanced, facilitating
the binding and also providing an additional mechanism
of electric modulation.
D. Headgroup Orientation in Glycolipids and
Glycosphingolipids and Their Influence on
Phospholipid Headgroups
The deuterium order parameter of headgroup-labeled
glycolipids and glycosphingolipids generally show a
headgroup orientation in which the sugar residues project
essentially straight up from the bilayer surface into the
aqueousregion,permittingmaximumhydrationoftheglu-
cose hydroxyl groups by water. The glucosyl headgroup
appears to be rather rigid, but rotates with a rotational
8 −1
diffusion constant of ∼10 s .
The headgroup conformational changes of deuterium-
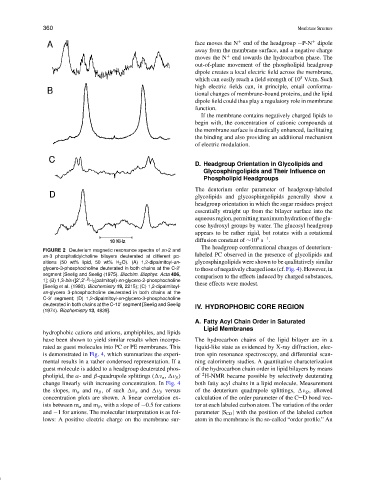
FIGURE 2 Deuterium magnetic resonance spectra of sn-2 and
labeled PC observed in the presence of glycolipids and
sn-3 phosphatidylcholine bilayers deuterated at different po-
sitions (50 wt% lipid, 50 wt% H 2 O). (A) 1,2-dipalmitoyl-sn- glycosphingolipids were shown to be qualitatively similar
glycero-3-phosphocholine deuterated in both chains at the C-2
to those of negatively charged ions (cf. Fig. 4). However, in
segment [Seelig and Seelig (1975). Biochim. Biophys. Acta 406, comparison to the effects induced by charged substances,
2
1]; (B) 1,3-bis-([2 ,2 - H 2 ]palmitoyl)-sn-glycero-2-phosphocholine
[Seelig et al. (1980). Biochemistry 19, 2215); (C) 1,2-dipalmitoyl- these effects were modest.
sn-glycero 3-phosphocholine deuterated in both chains at the
C-3 segment; (D) 1,2-dipalmitoyl-sn-glycero-3-phosphocholine
deuterated in both chains at the C-10 segment [Seelig and Seelig IV. HYDROPHOBIC CORE REGION
(1974). Biochemistry 13, 4839].
A. Fatty Acyl Chain Order in Saturated
Lipid Membranes
hydrophobic cations and anions, amphiphiles, and lipids
have been shown to yield similar results when incorpo- The hydrocarbon chains of the lipid bilayer are in a
rated as guest molecules into PC or PE membranes. This liquid-like state as evidenced by X-ray diffraction, elec-
is demonstrated in Fig. 4, which summarizes the experi- tron spin resonance spectroscopy, and differential scan-
mental results in a rather condensed representation. If a ning calorimetry studies. A quantitative characterization
guest molecule is added to a headgroup deuterated phos- of the hydrocarbon chain order in lipid bilayers by means
2
pholipid, the α- and β-quadrupole splittings ( ν α , ν β ) of H-NMR became possible by selectively deuterating
change linearly with increasing concentration. In Fig. 4 both fatty acyl chains in a lipid molecule. Measurement
the slopes, m α and m β , of such ν α and ν β versus of the deuterium quadrupole splittings, ν Q , allowed
concentration plots are shown. A linear correlation ex- calculation of the order parameter of the C D bond vec-
ists between m α and m β , with a slope of −0.5 for cations tor at each labeled carbon atom. The variation of the order
and −1 for anions. The molecular interpretation is as fol- parameter |S CD | with the position of the labeled carbon
lows: A positive electric charge on the membrane sur- atom in the membrane is the so-called “order profile.” An