Page 118 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 118
P1: GMY/GlQ/GLT P2: GRB Final Pages
Encyclopedia of Physical Science and Technology en010k-502 July 16, 2001 16:56
856 Nucleic Acid Synthesis
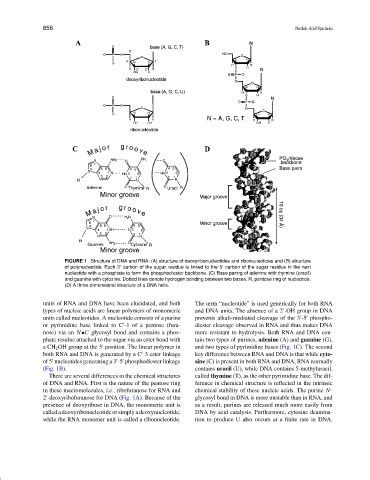
FIGURE 1 Structure of DNA and RNA: (A) structure of deoxyribonucleotides and ribonucleotides and (B) structure
of polynucleotide. Each 3 carbon of the sugar residue is linked to the 5 carbon of the sugar residue in the next
nucleotide with a phosphate to form the phosphodiester backbone. (C) Base paring of adenine with thymine (uracil)
and guanine with cytosine. Dotted lines denote hydrogen bonding between two bases. R, pentose ring of nucleotide.
(D) A three-dimensional structure of a DNA helix.
units of RNA and DNA have been elucidated, and both The term “nucleotide” is used generically for both RNA
types of nucleic acids are linear polymers of monomeric and DNA units. The absence of a 2 -OH group in DNA
units called nucleotides. A nucleotide consists of a purine prevents alkali-mediated cleavage of the 3 -5 phospho-
or pyrimidine base linked to C -1 of a pentose (fura- diester cleavage observed in RNA and thus makes DNA
nose) via an N•C glycosyl bond and contains a phos- more resistant to hydrolysis. Both RNA and DNA con-
phate residue attached to the sugar via an ester bond with tain two types of purines, adenine (A) and guanine (G),
aCH 2 OH group at the 5 position. The linear polymer in and two types of pyrimidine bases (Fig. 1C). The second
both RNA and DNA is generated by a C -3 ester linkage key difference between RNA and DNA is that while cyto-
of 5 nucleotides generating a 3 -5 phosphodiester linkage sine (C) is present in both RNA and DNA, RNA normally
(Fig. 1B). contains uracil (U), while DNA contains 5-methyluracil,
There are several differences in the chemical structures called thymine (T), as the other pyrimidine base. The dif-
of DNA and RNA. First is the nature of the pentose ring ference in chemical structure is reflected in the intrinsic
in these macromolecules, i.e., ribofuranose for RNA and chemical stability of these nucleic acids. The purine N-
2 -deoxyribofuranose for DNA (Fig. 1A). Because of the glycosyl bond in DNA is more unstable than in RNA, and
presence of deoxyribose in DNA, the monomeric unit is as a result, purines are released much more easily from
calledadeoxyribonucleotideorsimplyadeoxynucleotide, DNA by acid catalysis. Furthermore, cytosine deamina-
while the RNA monomer unit is called a ribonucleotide. tion to produce U also occurs at a finite rate in DNA.