Page 137 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 137
P1: GMY/GlQ/GLT P2: GRB Final Pages
Encyclopedia of Physical Science and Technology en010k-502 July 16, 2001 16:56
Nucleic Acid Synthesis 875
deoxyoligonucleotides with the possibility of automating between each reaction. The complete procedure has been
thecyclicprocedure.Onewasbasedonphosphodiestersof automated in several commercial instruments. After syn-
deoxynucleotides as the starting material, which had been thesisiscompleted,theoligonucleotideproductisreleased
utilized early on for synthesis of oligodeoxynucleotides. from the glass matrix by alkaline treatment, and then the
However, the phosphoramidite method invented later has last protective trityl group is removed. The quality and
become the exclusive method of choice for synthesis of efficiency of polymer synthesis is determined by the ef-
both RNA and DNA sequences. The advantages of this ficiency of the individual reactions. The major advantage
method are (1) the relatively high stability of the start- ofphosphoramidite-basedsynthesisisveryhighefficiency
ing compounds and (2) the mild reaction conditions for (99%) of both the condensation and the deprotection re-
removal of the protective groups. actions. Nonetheless, it is obvious that because the final
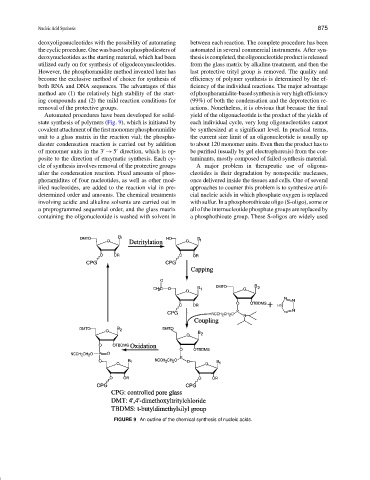
Automated procedures have been developed for solid- yield of the oligonucleotide is the product of the yields of
state synthesis of polymers (Fig. 9), which is initiated by each individual cycle, very long oligonucleotides cannot
covalent attachment ofthefirstmonomer phosphoramidite be synthesized at a significant level. In practical terms,
unit to a glass matrix in the reaction vial; the phospho- the current size limit of an oligonucleotide is usually up
diester condensation reaction is carried out by addition to about 120 monomer units. Even then the product has to
of monomer units in the 3 → 5 direction, which is op- be purified (usually by gel electrophoresis) from the con-
posite to the direction of enzymatic synthesis. Each cy- taminants, mostly composed of failed synthesis material.
cle of synthesis involves removal of the protective groups A major problem in therapeutic use of oligonu-
after the condensation reaction. Fixed amounts of phos- cleotides is their degradation by nonspecific nucleases,
phoramidites of four nucleotides, as well as other mod- once delivered inside the tissues and cells. One of several
ified nucleotides, are added to the reaction vial in pre- approaches to counter this problem is to synthesize artifi-
determined order and amounts. The chemical treatments cial nucleic acids in which phosphate oxygen is replaced
involving acidic and alkaline solvents are carried out in with sulfur. In a phosphorothioate oligo (S-oligo), some or
a preprogrammed sequential order, and the glass matrix all of the internucleotide phosphate groups are replaced by
containing the oligonucleotide is washed with solvent in a phosphothioate group. These S-oligos are widely used
FIGURE 9 An outline of the chemical synthesis of nucleic acids.