Page 39 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 39
P1: FPP Final
Encyclopedia of Physical Science and Technology EN006C-254 June 28, 2001 19:52
Food Colors 111
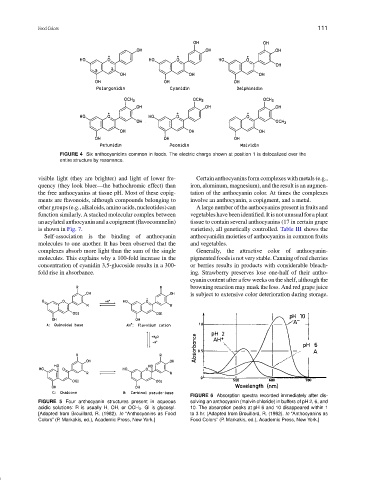
FIGURE 4 Six anthocyanidins common in foods. The electric charge shown at position 1 is delocalized over the
entire structure by resonance.
visible light (they are brighter) and light of lower fre- Certain anthocyanins form complexes with metals (e.g.,
quency (they look bluer—the bathochromic effect) than iron, aluminum, magnesium), and the result is an augmen-
the free anthocyanins at tissue pH. Most of these copig- tation of the anthocyanin color. At times the complexes
ments are flavonoids, although compounds belonging to involve an anthocyanin, a copigment, and a metal.
other groups (e.g., alkaloids, amino acids,nucleotides) can A large number of the anthocyanins present in fruits and
function similarly. A stacked molecular complex between vegetables have been identified. It is not unusual for a plant
an acylated anthocyanin and a copigment (flavocommelin) tissue to contain several anthocyanins (17 in certain grape
is shown in Fig. 7. varieties), all genetically controlled. Table III shows the
Self-association is the binding of anthocyanin anthocyanidin moieties of anthocyanins in common fruits
molecules to one another. It has been observed that the and vegetables.
complexes absorb more light than the sum of the single Generally, the attractive color of anthocyanin-
molecules. This explains why a 100-fold increase in the pigmented foods is not very stable. Canning of red cherries
concentration of cyanidin 3,5-glucoside results in a 300- or berries results in products with considerable bleach-
fold rise in absorbance. ing. Strawberry preserves lose one-half of their antho-
cyanin content after a few weeks on the shelf, although the
browning reaction may mask the loss. And red grape juice
is subject to extensive color deterioration during storage.
FIGURE 6 Absorption spectra recorded immediately after dis-
FIGURE 5 Four anthocyanin structures present in aqueous solving an anthocyanin (malvin chloride) in buffers of pH 2, 6, and
acidic solutions: R is usually H, OH, or OCH 3 . Gl is glycosyl. 10. The absorption peaks at pH 6 and 10 disappeared within 1
[Adapted from Brouillard, R. (1982). In “Anthocyanins as Food to 3 hr. (Adapted from Brouillard, R. (1982). In “Anthocyanins as
Colors” (P. Markakis, ed.), Academic Press, New York.] Food Colors” (P. Markakis, ed.), Academic Press, New York.]