Page 16 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 16
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
220 Biopolymers
paper-making, but have not yet been fully developed as a These two forms (anomeric forms) are distinguished as an
resource. α- or β-sugar. In the D-series of sugars, the α-form has the
anomeric hydroxyl lying below the plane of the ring, while
in the L-sugars, this hydroxyl lies above the ring plane in
1. Structure
the α-form. The full name of a monosaccharide indicates
As stated above, the monomers of polysaccharides are whether the sugar is α or β, D or L, which kind of sugar
monosaccharides or sugars. Most of these have the gen- is being dealt with, and the size of the sugar ring, hence
eral formula (CH 2 O) x , and were considered initially as the names α-D-glucopyranose and β-D-mannopyranose in
hydrates of carbon, hence the name carbohydrates. The Fig. 8a.
most common monomers have five carbon atoms (the pen- Neither the furanose nor pyranose rings are flat, but
toses) or six carbon atoms (the hexoses). Each monosac- are puckered. The most stable conformations of pyranose
4
charide has many hydroxyl (OH) groups on the molecule, sugars are “chair” forms, and these are designated C 1 or
and one monosaccharide differs from another in the ar- 1 C 4 ,dependingonwhethertheanomericcarbon(carbon1)
rangement in space of the hydroxyl groups. In solution is at a top or bottom apex (Fig. 8). (Monosaccharides with
a monosaccharide can exist in a number of forms. The six-memberedringsmayberepresentedeitherbythe“flat”
monosaccharides can be thought of as carrying an alde- projection of the pyranose ring shown in Fig. 8a, or by
hyde or ketone group, and hence have reducing character. the chair forms more closely related to three-dimensional
However, intra-molecular cyclization of a monosaccha- shape shown in Fig 8b.)
ride can occur to give a ring form, known as a hemiacetal A wide variety of monosaccharides can be incorporated
or hemiketal, where the original aldehyde or ketone group into polysaccharides and some of the most common are
is masked by reaction with a hydroxyl group of the same shown in Fig. 8. Not all monosaccharides have the gen-
molecule. This cyclization gives a six-membered ring eral formula (CH 2 O) x . Some have one oxygen less; these
form (pyranose) or a five-membered ring form (furanose) are the deoxy sugars (Fig. 8d). Others have a substituted
(Fig. 8). Usually only one ring form of a monosaccharide amino (NH 2 ) group usually on carbon 2; these are the
is found in any one polysaccharide. Each monosaccha- amino sugars (Fig. 8e). Others may carry sulfate groups;
ride can exist in two mirror-image forms, the D- and L- yet others have acid groups (COOH) instead of CH 2 OH
forms just as amino acids are found in D- and L-forms. at carbon 6; these are the uronic acids (Fig. 8f ).
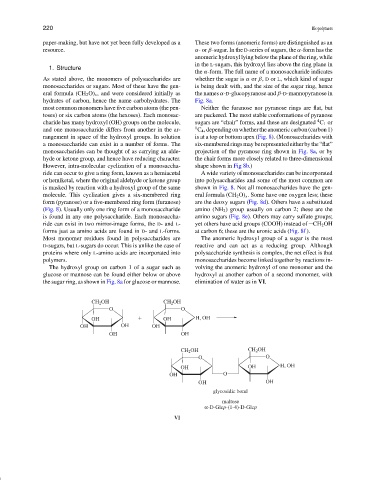
Most monomer residues found in polysaccharides are The anomeric hydroxyl group of a sugar is the most
D-sugars, but L-sugars do occur. This is unlike the case of reactive and can act as a reducing group. Although
proteins where only L-amino acids are incorporated into polysaccharide synthesis is complex, the net effect is that
polymers. monosaccharides become linked together by reactions in-
The hydroxyl group on carbon 1 of a sugar such as volving the anomeric hydroxyl of one monomer and the
glucose or mannose can be found either below or above hydroxyl at another carbon of a second monomer, with
the sugar ring, as shown in Fig. 8a for glucose or mannose. elimination of water as in VI.
CH 2 OH CH 2 OH
O O
OH OH H, OH
OH OH OH
OH OH
CH 2 OH CH 2 OH
O O
OH OH H, OH
OH O
OH OH
glycosidic bond
maltose
α-D-Glcp-(1-4)-D-Glcp
VI