Page 18 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 18
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
222 Biopolymers
The product is a disaccharide, in which two monosac-
charide residues are linked by a glycosidic bond. In VI
the reaction has taken place between an α-hydroxyl on
carbon 1 of one glucose and a hydroxyl on carbon 4 of
the second monosaccharide. The new bond is therefore
an α-(1 → 4)-glycosidic bond. Reaction can, in fact, take
place to link carbon 1 of one sugar through any of the
free hydroxyl groups attached to carbons 2, 3, 4, or 6 on
a second sugar, for example, glucose in the pyranose ring
form, yielding 1 → 2, 1 → 3, 1 → 4, or 1 → 6 glycosidic
bonds, respectively. In addition, the bond is designated
as an α-or β-bond, depending on whether the anomeric
hydroxyl of the first sugar lay originally below or above
the plane of the monosaccharide ring. Many disaccha-
rides have “trivial” (here maltose) and systematic names.
An abbreviated form of the systematic name of maltose
is shown. The sugar with the free hydroxyl on carbon 1
(the reducing end) is always written at the right of a dis-
accharide or polysaccharide, and the other monosaccha-
ride residues are written to the left of it. In the systematic
name for a disaccharide or polysaccharide, the name of the
left-hand sugar is given first (here we have α–D–Glcp).
Then the type of glycosidic linkage (here l → 4) is indi-
cated, followed by the name of the next sugar. Using com-
mon abbreviations the maltose structure can be written as
α-D-Glcp-(1 → 4)-D-Glcp.
Besides the hydroxyl on carbon 1 of the right hand
sugar, there are seven other hydroxyl groups which could,
in theory, react with the carbon 1 hydroxyl of a third
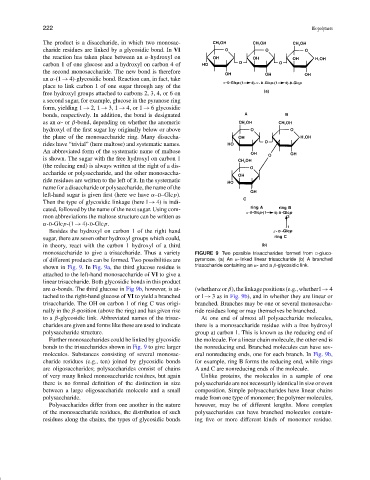
monosaccharide to give a trisaccharide. Thus a variety FIGURE 9 Two possible trisaccharides formed from D-gluco-
of different products can be formed. Two possibilities are pyranose. (a) An α-linked linear trisaccharide (b) A branched
shown in Fig. 9. In Fig. 9a, the third glucose residue is trisaccharide containing an α- and a β-glycosidic link.
attached to the left-hand monosaccharide of VI to give a
linear trisaccharide. Both glycosidic bonds in this product
are α-bonds. The third glucose in Fig 9b, however, is at- (whether α or β),thelinkagepositions(e.g.,whetherl → 4
tached to the right-hand glucose of VI to yield a branched or l → 3 as in Fig. 9b), and in whether they are linear or
trisaccharide. The OH on carbon 1 of ring C was origi- branched. Branches may be one or several monosaccha-
nally in the β-position (above the ring) and has given rise ride residues long or may themselves be branched.
to a β-glycosidic link. Abbreviated names of the trisac- At one end of almost all polysaccharide molecules,
charides are given and forms like these are used to indicate there is a monosaccharide residue with a free hydroxyl
polysaccharide structure. group at carbon 1. This is known as the reducing end of
Further monosaccharides could be linked by glycosidic the molecule. For a linear chain molecule, the other end is
bonds to the trisaccharides shown in Fig. 9 to give larger the nonreducing end. Branched molecules can have sev-
molecules. Substances consisting of several monosac- eral nonreducing ends, one for each branch. In Fig. 9b,
charide residues (e.g., ten) joined by glycosidic bonds for example, ring B forms the reducing end, while rings
are oligosaccharides; polysaccharides consist of chains A and C are nonreducing ends of the molecule.
of very many linked monosaccharide residues, but again Unlike proteins, the molecules in a sample of one
there is no formal definition of the distinction in size polysaccharide are not necessarily identical in size or even
between a large oligosaccharide molecule and a small composition. Simple polysaccharides have linear chains
polysaccharide. made from one type of monomer; the polymer molecules,
Polysaccharides differ from one another in the nature however, may be of different lengths. More complex
of the monosaccharide residues, the distribution of such polysaccharides can have branched molecules contain-
residues along the chains, the types of glycosidic bonds ing five or more different kinds of monomer residue.