Page 249 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 249
P1: GNH/GRI P2: GTV Final pages
Encyclopedia of Physical Science and Technology EN012B-596 July 27, 2001 18:18
758 Polymers, Synthesis
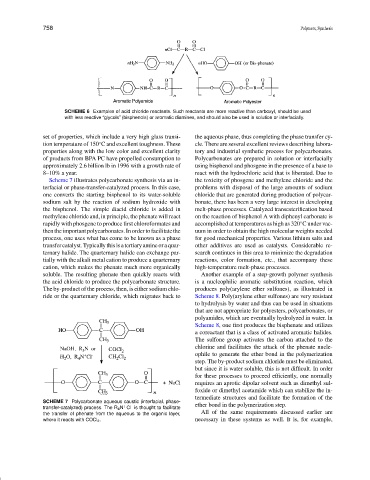
SCHEME 6 Examples of acid chloride reactants. Such reactants are more reactive than carboxyl, should be used
with less reactive “glycols” (bisphenols) or aromatic diamines, and should also be used in solution or interfacially.
set of properties, which include a very high glass transi- the aqueous phase, thus completing the phase transfer cy-
◦
tion temperature of 150 C and excellent toughness. These cle. There are several excellent reviews describing labora-
properties along with the low color and excellent clarity tory and industrial synthetic process for polycarbonates.
of products from BPA PC have propelled consumption to Polycarbonates are prepared in solution or interfacially
approximately 2.6 billion lb in 1996 with a growth rate of using bisphenol and phosgene in the presence of a base to
8–10% a year. react with the hydrochloric acid that is liberated. Due to
Scheme 7 illustrates polycarbonate synthesis via an in- the toxicity of phosgene and methylene chloride and the
terfacial or phase-transfer-catalyzed process. In this case, problems with disposal of the large amounts of sodium
one converts the starting bisphenol to its water-soluble chloride that are generated during production of polycar-
sodium salt by the reaction of sodium hydroxide with bonate, there has been a very large interest in developing
the bisphenol. The simple diacid chloride is added in melt-phase processes. Catalyzed transesterification based
methylene chloride and, in principle, the phenate will react on the reaction of bisphenol A with diphenyl carbonate is
◦
rapidly with phosgene to produce first chloroformates and accomplished at temperatures as high as 320 C under vac-
then the important polycarbonates. In order to facilitate the uum in order to obtain the high molecular weights needed
process, one uses what has come to be known as a phase for good mechanical properties. Various lithium salts and
transfercatalyst.Typicallythisisatertiaryamineoraquar- other additives are used as catalysts. Considerable re-
ternary halide. The quarternary halide can exchange par- search continues in this area to minimize the degradation
tially with the alkali metal cation to produce a quarternary reactions, color formation, etc., that accompany these
cation, which makes the phenate much more organically high-temperature melt-phase processes.
soluble. The resulting phenate then quickly reacts with Another example of a step-growth polymer synthesis
the acid chloride to produce the polycarbonate structure. is a nucleophilic aromatic substitution reaction, which
The by-product of the process, then, is either sodium chlo- produces poly(arylene ether sulfones), as illustrated in
ride or the quarternary chloride, which migrates back to Scheme 8. Poly(arylene ether sulfones) are very resistant
to hydrolysis by water and thus can be used in situations
that are not appropriate for polyesters, polycarbonates, or
polyamides, which are eventually hydrolyzed in water. In
Scheme 8, one first produces the bisphenate and utilizes
a coreactant that is a class of activated aromatic halides.
The sulfone group activates the carbon attached to the
chlorine and facilitates the attack of the phenate nucle-
ophile to generate the ether bond in the polymerization
step. The by-product sodium chloride must be eliminated,
but since it is water soluble, this is not difficult. In order
for these processes to proceed efficiently, one normally
requires an aprotic dipolar solvent such as dimethyl sul-
foxide or dimethyl acetamide which can stabilize the in-
termediate structures and facilitate the formation of the
SCHEME 7 Polycarbonate aqueous caustic (interfacial, phase-
+
−
transfer-catalyzed) process. The R 4 N Cl is thought to facilitate ether bond in the polymerization step.
the transfer of phenate from the aqueous to the organic layer, All of the same requirements discussed earlier are
where it reacts with COCl 2 . necessary in these systems as well. It is, for example,