Page 250 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 250
P1: GNH/GRI P2: GTV Final pages
Encyclopedia of Physical Science and Technology EN012B-596 July 27, 2001 18:18
Polymers, Synthesis 759
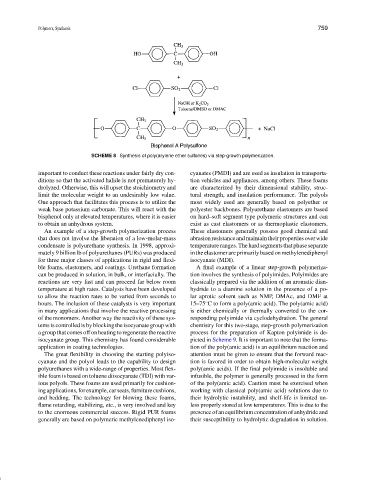
SCHEME 8 Synthesis of poly(arylene ether sulfones) via step-growth polymerization.
important to conduct these reactions under fairly dry con- cyanates (PMDI) and are used as insulation in transporta-
ditions so that the activated halide is not prematurely hy- tion vehicles and appliances, among others. These foams
drolyzed. Otherwise, this will upset the stoichiometry and are characterized by their dimensional stability, struc-
limit the molecular weight to an undesirably low value. tural strength, and insulation performance. The polyols
One approach that facilitates this process is to utilize the most widely used are generally based on polyether or
weak base potassium carbonate. This will react with the polyester backbones. Polyurethane elastomers are based
bisphenol only at elevated temperatures, where it is easier on hard–soft segment type polymeric structures and can
to obtain an anhydrous system. exist as cast elastomers or as thermoplastic elastomers.
An example of a step-growth polymerization process These elastomers generally possess good chemical and
that does not involve the liberation of a low-molar-mass abrasionresistanceandmaintaintheirpropertiesoverwide
condensate is polyurethane synthesis. In 1998, approxi- temperature ranges. The hard segments that phase separate
mately 9 billion lb of polyurethanes (PURs) was produced intheelastomerareprimarilybasedonmethylenediphenyl
for three major classes of applications in rigid and flexi- isocyanate (MDI).
ble foams, elastomers, and coatings. Urethane formation A final example of a linear step-growth polymeriza-
can be produced in solution, in bulk, or interfacially. The tion involves the synthesis of polyimides. Polyimides are
reactions are very fast and can proceed far below room classically prepared via the addition of an aromatic dian-
temperature at high rates. Catalysts have been developed hydride to a diamine solution in the presence of a po-
to allow the reaction rates to be varied from seconds to lar aprotic solvent such as NMP, DMAc, and DMF at
hours. The inclusion of these catalysts is very important 15–75 C to form a poly(amic acid). The poly(amic acid)
◦
in many applications that involve the reactive processing is either chemically or thermally converted to the cor-
of the monomers. Another way the reactivity of these sys- responding polyimide via cyclodehydration. The general
tems is controlled is by blocking the isocyanate group with chemistry for this two-stage, step-growth polymerization
a group that comes off on heating to regenerate the reactive process for the preparation of Kapton polyimide is de-
isocyanate group. This chemistry has found considerable picted in Scheme 9. It is important to note that the forma-
application in coating technologies. tion of the poly(amic acid) is an equilibrium reaction and
The great flexibility in choosing the starting polyiso- attention must be given to ensure that the forward reac-
cyanate and the polyol leads to the capability to design tion is favored in order to obtain high-molecular weight
polyurethanes with a wide-range of properties. Most flex- poly(amic acids). If the final polyimide is insoluble and
ible foam is based on toluene diisocyanate (TDI) with var- infusible, the polymer is generally processed in the form
ious polyols. These foams are used primarily for cushion- of the poly(amic acid). Caution must be exercised when
ingapplications,forexample,carseats,furniturecushions, working with classical poly(amic acid) solutions due to
and bedding. The technology for blowing these foams, their hydrolytic instability, and shelf-life is limited un-
flame retarding, stabilizing, etc., is very involved and key less properly stored at low temperatures. This is due to the
to the enormous commercial success. Rigid PUR foams presence of an equilibrium concentration of anhydride and
generally are based on polymeric methylenediphenyl iso- their susceptibility to hydrolytic degradation in solution.